Abstract
Purpose
Our aim was to describe all serious cutaneous adverse drug reactions (ADRs) spontaneously reported in France for all oral protein kinase inhibitors, their characteristics and whether they were labeled (reported in the Summary of Product Characteristics) or not.
Methods
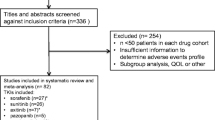
We performed a retrospective observational study in the French PharmacoVigilance Database, selecting for analysis serious cutaneous reactions of patients due to treatment with oral protein kinase inhibitors (erlotinib, gefitinib, imatinib, nilotinib, dasatinib, sunitinib, sorafenib, pazopanib, lapatinib, everolimus) between 1 January 2008 and 31 December 31 2010.
Results
Ninety-four patients suffered from 115 serious cutaneous reactions due to oral protein kinase inhibitors. Serious cutaneous reactions more frequently reported were maculo-papular rash (mostly with imatinib), followed by hand–foot syndrome (specifically with sorafenib) and papulopustular rash (particularly with erlotinib). Patients were mostly males (63 %) with a mean age of 62.6 ± 15.4 years. Drug withdrawal was observed in 73.1 % of cases because of these cutaneous reactions. Delay of occurrence of the ADR varied from 11.5 to 58.5 days. Unlabeled serious reactions were found (17.4 %), including skin ulceration, vasculitis or purpura with sorafenib or sunitinib and drug rash with eosinophilia and systemic symptoms with imatinib.
Conclusion
Some of the serious ADRs spontaneously reported with oral protein kinase inhibitors are labeled and commonly reported in the literature, but others occur only rarely and unlabeled. In our study, most serious ADRs occurred in males within the 2 first months of treatment and were responsible for the withdrawal of therapy with protein kinase inhibitors.


Similar content being viewed by others
References
Castot A, Haramburu F, Kreft-Jaïs C (2008) Hospitalisations dues aux effets indésirables des médicaments: résultats d’une étude nationale. Point sur la nouvelle campagne d’information sur les traitements anticoagulants antivitamine K. Available at: http://www.sante.gouv.fr/IMG/pdf/EMIR.pdf. Accessed 4 Jan 2013
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet 356:1255–1259
Lynch TJ Jr, Kim ES, Eaby B et al (2007) Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist 12:610–621
Amitay-Laish I, Stemmer SM, Lacouture ME (2011) Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol Ther 24:386–395
Lee WJ, Lee JL, Chang SE et al (2009) Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol 161:1045–1051
Bible KC, Suman VJ, Molina JR et al (2010) Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11:962–972
Motzer RJ, Escudier B, Oudard S et al (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116:4256–4265
Montastruc J-L, Sommet A, Lacroix I et al (2006) Pharmacovigilance for evaluating adverse drug reactions: value, organization, and methods. Joint Bone Spine 73:629–632
Spreux A, Baldin B, Chichmanian RM (1999) Pharmacovigilance in practice. Transfus Clin Biol 6:254–259
Goldfarb N (2012) Adverse event terminology. J Clin Res Best Pract 8:1–17
Brown EG, Wood L, Wood S (1999) The medical dictionary for regulatory activities (MedDRA). Drug Saf 20:109–117
Bégaud B, Evreux JC, Jouglard J et al (1985) Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 40:111–118
Heidary N, Naik H, Burgin S (2008) Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol 58:545–570
Robert C, Soria J-C, Spatz A et al (2005) Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6:491–500
Pierfitte C, Bégaud B, Lagnaoui R et al (1999) Is reporting rate a good predictor of risks associated with drugs? Br J Clin Pharmacol 47:329–331
Van der Heijden PGM, Van Puijenbroek EP, Van Buuren S et al (2007) On the assessment of adverse drug reactions from spontaneous reporting systems: the influence of under-reporting on odds ratios. Stat Med 21:2027–2044
Bégaud B, Martin K, Haramburu F et al (2002) Rates of spontaneous reporting of adverse drug reactions in France. JAMA 288:1588
Théophile H, Arimone Y, Miremont-Salamé G et al (2010) Comparison of three methods (consensual expert judgement, algorithmic and probabilistic approaches) of causality assessment of adverse drug reactions: an assessment using reports made to a French pharmacovigilance centre. Drug Saf 33:1045–1054
Valeyrie L, Bastuji-Garin S, Revuz J et al (2003) Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: a prospective study of 54 patients. J Am Acad Dermatol 48:201–206
Choi MK, Woo HY, Heo J et al (2011) Toxic epidermal necrolysis associated with sorafenib and tosufloxacin in a patient with hepatocellular carcinoma. Ann Dermatol 23[Suppl 3]:S404–S407
Dean SM, Zirwas M (2010) A second case of sunitinib-associated pyoderma gangrenosum. J Clin Aesthet Dermatol 3:34–35
Ten Freyhaus K, Homey B, Bieber T et al (2008) Pyoderma gangrenosum: another cutaneous side-effect of sunitinib? Br J Dermatol 159:242–243
Karadimou A, Migou M, Economidi A et al (2011) Leukocytoclastic vasculitis after long-term treatment with sunitinib: a case report. Case Rep Oncol 4:385–391
Chung NM, Gutierrez M, Turner ML (2006) Leukocytoclastic vasculitis masquerading as hand-foot syndrome in a patient treated with sorafenib. Arch Dermatol 142:1510–1511
Najarian DJ, Packianathan V, Zeitouni NC (2010) Annular leukocytoclastic vasculitis associated with sorafenib administration. J Drugs Dermatol 9:697–698
Trinkaus M, Trudeau M, Callum J (2008) Drug-induced immune thrombocytopenic purpura secondary to sunitinib. Curr Oncol 15:152–154
Goldman J, Duval-Modeste A-B, Lambert A et al (2008) Imatinib-induced DRESS. Ann Dermatol Venereol 135:393–396
Le Nouail P, Viseux V, Chaby G et al (2006) Drug reaction with eosinophilia and systemic symptoms (DRESS) following imatinib therapy. Ann Dermatol Venereol 133:686–688
Benomar S, Boutayeb S, Afifi Y et al (2009) Hand-foot syndrome and seborrheic dermatitis-like eruption induced by erlotinib. Dermatol Online J 15:2
Takahashi Y, Ebi N, Yamaguchi O et al (2011) A case of cutaneous vasculitis caused by erlotinib treatment and a review of literature. Nihon Kokyuki Gakkai Zasshi 49:663–666
Pitarch G, Garde J, Torrijos A et al (2008) Adverse cutaneous reactions to erlotinib. Actas Dermosifiliogr 99:54–60
National Institute of Health and National Cancer Institute (2010) Common terminology criteria for adverse events (CTCAE) v4.03. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 4 Jan 2013
Nikolaou V, Stratigos A, Antoniou C et al (2010) Pimecrolimus cream 1 % for the treatment of papulopustular eruption related to epidermal growth factor receptor inhibitors: a case series and a literature review of therapeutic approaches. Dermatology (Basel) 220:243–248
Anderson R, Jatoi A, Robert C et al (2009) Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist 14:291–302
European Medicines Agency (2012) Tarceva: summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000618/WC500033994.pdf. Accessed 4 Jan 2013
European Medicines Agency (2013) Sutent: summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000687/WC500057737.pdf. Accessed 28 Jan 2013
Statement of all funding sources
None
Conflict of interest disclosure
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Faye, E., Bondon-Guitton, E., Olivier-Abbal, P. et al. Spontaneous reporting of serious cutaneous reactions with protein kinase inhibitors. Eur J Clin Pharmacol 69, 1819–1826 (2013). https://doi.org/10.1007/s00228-013-1532-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1532-6