Abstract
Objectives
The main aim of this study was to determine whether CYP2C9 and VKORC1 polymorphisms influence warfarin dose variability during initial dose-finding phase and during maintenance treatment after 360 days.
Methods
Two hundred and six consecutive patients who were beginning warfarin therapy were selected. They were assessed for general and clinical characteristics; prescribed warfarin dose; response to therapy on days 7–10, 30, 60, 180, and 360; adverse events; and CYP2C9 *2, *3, *5, *6, *8, *11, and VKORC1 1639G >A assays.
Results
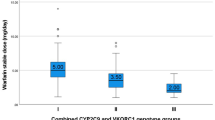
During the first 30 days of anticoagulation, the relative variability of warfarin dose was significantly associated with CYP2C9*2 and CYP2C9*3 polymorphisms (p = 0.02) and with VKORC1 1639G >A genotypes (p = 0.04). Warfarin variability was also statistically different according to predicted metabolic phenotype and to VKORC1 genotypes after 360 days of treatment, and in the phase between 180 and 360 days (long-term dose variability). Both CYP2C9 and VKORC1 polymorphisms were associated with the international normalized ratio (INR) made between 7 and 10 days/initial dose ratio, adjusted for covariates (p < 0.01 and p = 0.02, respectively). Patients carrying VKORC1 and CYP2C9 variants presented lower required dose (at the end of follow-up of 360 days) compared to patients carrying wild-type genotypes (p = 0.04 and p = 0.03, respectively).
Conclusions
Genetic information on CYP2C9 and VKORC1 is important both for the initial dose-finding phase and during maintenance treatment with warfarin.


Similar content being viewed by others
References
Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D (2001) Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 119(1 Suppl):8S–21S
Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D, Brandt JT (1998) Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 114(5 Suppl):445S–469S
van den Besselaar AM (1985) Standardization of the prothrombin time in oral anticoagulant control. Haemostasis 15(4):271–277
Gutierrez C, Blanchard DG Atrial fibrillation: diagnosis and treatment. Am Fam Physician 83(1):61–68
Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B (1989) Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet 1(8631):175–179
Landefeld CS, Beyth RJ (1993) Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 95(3):315–328
Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, Kim RB, Roden DM, Stein CM (2008) Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med 358(10):999–1008. doi:10.1056/NEJMoa0708078
Johnson JA, Cavallari LH, Beitelshees AL, Lewis JP, Shuldiner AR, Roden DM (2011) Pharmacogenomics: application to the management of cardiovascular disease. Clin Pharmacol Ther 90(4):519–531. doi:10.1038/clpt.2011.179
Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, Wallerman O, Melhus H, Wadelius C, Bentley D, Deloukas P (2005) Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J 5(4):262–270. doi:10.1038/sj.tpj.6500313
Ferder NS, Eby CS, Deych E, Harris JK, Ridker PM, Milligan PE, Goldhaber SZ, King CR, Giri T, McLeod HL, Glynn RJ, Gage BF (2010) Ability of VKORC1 and CYP2C9 to predict therapeutic warfarin dose during the initial weeks of therapy. J Thromb Haemost 8(1):95–100. doi:10.1111/j.1538-7836.2009.03677.x
Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM, Rettie AE (2002) Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 287(13):1690–1698
Cavallari LH, Langaee TY, Momary KM, Shapiro NL, Nutescu EA, Coty WA, Viana MA, Patel SR, Johnson JA (2010) Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin Pharmacol Ther 87(4):459–464. doi:10.1038/clpt.2009.223
Scott SA, Jaremko M, Lubitz SA, Kornreich R, Halperin JL, Desnick RJ (2009) CYP2C9*8 is prevalent among African-Americans: implications for pharmacogenetic dosing. Pharmacogenomics 10(8):1243–1255. doi:10.2217/pgs.09.71
Geisen C, Watzka M, Sittinger K, Steffens M, Daugela L, Seifried E, Muller CR, Wienker TF, Oldenburg J (2005) VKORC1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thromb Haemost 94(4):773–779. doi:10.1160/TH05-04-0290
Li T, Lange LA, Li X, Susswein L, Bryant B, Malone R, Lange EM, Huang TY, Stafford DW, Evans JP (2006) Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. J Med Genet 43(9):740–744. doi:10.1136/jmg.2005.040410
Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352(22):2285–2293. doi:10.1056/NEJMoa044503
Kirchheiner J, Ufer M, Walter EC, Kammerer B, Kahlich R, Meisel C, Schwab M, Gleiter CH, Rane A, Roots I, Brockmoller J (2004) Effects of CYP2C9 polymorphisms on the pharmacokinetics of R- and S-phenprocoumon in healthy volunteers. Pharmacogenetics 14(1):19–26
Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, Lu MJ, Hung CR, Wei CY, Chen CH, Wu JY, Chen YT (2005) A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet 14(13):1745–1751. doi:10.1093/hmg/ddi180
Caldwell MD, Awad T, Johnson JA, Gage BF, Falkowski M, Gardina P, Hubbard J, Turpaz Y, Langaee TY, Eby C, King CR, Brower A, Schmelzer JR, Glurich I, Vidaillet HJ, Yale SH, Qi Zhang K, Berg RL, Burmester JK (2008) CYP4F2 genetic variant alters required warfarin dose. Blood 111(8):4106–4112. doi:10.1182/blood-2007-11-122010
Loebstein R, Dvoskin I, Halkin H, Vecsler M, Lubetsky A, Rechavi G, Amariglio N, Cohen Y, Ken-Dror G, Almog S, Gak E (2007) A coding VKORC1 Asp36Tyr polymorphism predisposes to warfarin resistance. Blood 109(6):2477–2480. doi:10.1182/blood-2006-08-038984
Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, Wood P, Kesteven P, Daly AK, Kamali F (2005) The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106(7):2329–2333. doi:10.1182/blood-2005-03-1108
Wu AH (2007) Use of genetic and nongenetic factors in warfarin dosing algorithms. Pharmacogenomics 8(7):851–861. doi:10.2217/14622416.8.7.851
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16(3):1215
Jetter A, Kinzig-Schippers M, Skott A, Lazar A, Tomalik-Scharte D, Kirchheiner J, Walchner-Bonjean M, Hering U, Jakob V, Rodamer M, Jabrane W, Kasel D, Brockmoller J, Fuhr U, Sorgel F (2004) Cytochrome P450 2C9 phenotyping using low-dose tolbutamide. Eur J Clin Pharmacol 60(3):165–171. doi:10.1007/s00228-004-0754-z
Santos PC, Soares RA, Krieger JE, Guerra-Shinohara EM, Pereira AC (2011) Genotyping of the hemochromatosis HFE p.H63D and p.C282Y mutations by high-resolution melting with the Rotor-Gene 6000((R)) instrument. Clin Chem Lab Med 49(10):1633–1636. doi:10.1515/CCLM.2011.654
Santos PC, Soares RA, Santos DB, Nascimento RM, Coelho GL, Nicolau JC, Mill JG, Krieger JE, Pereira AC (2011) CYP2C19 and ABCB1 gene polymorphisms are differently distributed according to ethnicity in the Brazilian general population. BMC Med Genet 12:13. doi:10.1186/1471-2350-12-13
Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ (2010) Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 11(6):781–791. doi:10.2217/pgs.10.49
Alvim RO, Freitas SR, Ferreira NE, Santos PC, Cunha RS, Mill JG, Krieger JE, Pereira AC (2010) APOE polymorphism is associated with lipid profile, but not with arterial stiffness in the general population. Lipids Health Dis 9:128. doi:10.1186/1476-511X-9-128
Kitzmiller JP, Groen DK, Phelps MA, Sadee W (2011) Pharmacogenomic testing: relevance in medical practice: why drugs work in some patients but not in others. Cleve Clin J Med 78(4):243–257. doi:10.3949/ccjm.78a.10145
Limdi NA, Wadelius M, Cavallari L, Eriksson N, Crawford DC, Lee MT, Chen CH, Motsinger-Reif A, Sagreiya H, Liu N, Wu AH, Gage BF, Jorgensen A, Pirmohamed M, Shin JG, Suarez-Kurtz G, Kimmel SE, Johnson JA, Klein TE, Wagner MJ (2010) Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across three racial groups. Blood 115(18):3827–3834. doi:10.1182/blood-2009-12-255992
Lindh JD, Lundgren S, Holm L, Alfredsson L, Rane A (2005) Several-fold increase in risk of overanticoagulation by CYP2C9 mutations. Clin Pharmacol Ther 78(5):540–550. doi:10.1016/j.clpt.2005.08.006
Molden E, Okkenhaug C, Ekker Solberg E (2010) Increased frequency of CYP2C9 variant alleles and homozygous VKORC1*2B carriers in warfarin-treated patients with excessive INR response. Eur J Clin Pharmacol 66(5):525–530. doi:10.1007/s00228-010-0813-6
Peyvandi F, Spreafico M, Siboni SM, Moia M, Mannucci PM (2004) CYP2C9 genotypes and dose requirements during the induction phase of oral anticoagulant therapy. Clin Pharmacol Ther 75(3):198–203. doi:10.1016/j.clpt.2003.09.015
Aithal GP, Day CP, Kesteven PJ, Daly AK (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 353(9154):717–719. doi:10.1016/S0140-6736(98)04474-2
Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, Milligan PE, Grice G, Lenzini P, Rettie AE, Aquilante CL, Grosso L, Marsh S, Langaee T, Farnett LE, Voora D, Veenstra DL, Glynn RJ, Barrett A, McLeod HL (2008) Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther 84(3):326–331. doi:10.1038/clpt.2008.10
Limdi NA, McGwin G, Goldstein JA, Beasley TM, Arnett DK, Adler BK, Baird MF, Acton RT (2008) Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther 83(2):312–321. doi:10.1038/sj.clpt.6100290
Epstein RS, Moyer TP, Aubert RE DJOK, Xia F, Verbrugge RR, Gage BF, Teagarden JR (2010) Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol 55(25):2804–2812. doi:10.1016/j.jacc.2010.03.009
Meckley LM, Wittkowsky AK, Rieder MJ, Rettie AE, Veenstra DL (2008) An analysis of the relative effects of VKORC1 and CYP2C9 variants on anticoagulation related outcomes in warfarin-treated patients. Thromb Haemost 100(2):229–239
Miao L, Yang J, Huang C, Shen Z (2007) Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur J Clin Pharmacol 63(12):1135–1141. doi:10.1007/s00228-007-0381-6
Perini JA, Struchiner CJ, Silva-Assuncao E, Santana IS, Rangel F, Ojopi EB, Dias-Neto E, Suarez-Kurtz G (2008) Pharmacogenetics of warfarin: development of a dosing algorithm for Brazilian patients. Clin Pharmacol Ther 84(6):722–728. doi:10.1038/clpt.2008.166
Botton MR, Bandinelli E, Rohde LE, Amon LC, Hutz MH (2011) Influence of genetic, biological and pharmacological factors on warfarin dose in a Southern Brazilian population of European ancestry. Br J Clin Pharmacol 72(3):442–450. doi:10.1111/j.1365-2125.2011.03942.x
You JH (2011) Pharmacoeconomic evaluation of warfarin pharmacogenomics. Expert Opin Pharmacother 12(3):435–441. doi:10.1517/14656566.2011.521153
Ginsburg GS, Voora D (2011) The long and winding road to warfarin pharmacogenetic testing. J Am Coll Cardiol 55(25):2813–2815. doi:10.1016/j.jacc.2010.04.006
Finkelman BS, Gage BF, Johnson JA, Brensinger CM, Kimmel SE (2011) Genetic warfarin dosing: tables versus algorithms. J Am Coll Cardiol 57(5):612–618. doi:10.1016/j.jacc.2010.08.643
Huang SW, Chen HS, Wang XQ, Huang L, Xu DL, Hu XJ, Huang ZH, He Y, Chen KM, Xiang DK, Zou XM, Li Q, Ma LQ, Wang HF, Chen BL, Li L, Jia YK, Xu XM (2009) Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenet Genomics 19(3):226–234. doi:10.1097/FPC.0b013e328326e0c7
Pavani A, Naushad SM, Rupasree Y, Kumar TR, Malempati AR, Pinjala RK, Mishra RC, Kutala VK (2011) Optimization of warfarin dose by population-specific pharmacogenomic algorithm. Pharmacogenomics J. doi:10.1038/tpj.2011.4
Zhong SL, Yu XY, Liu Y, Xu D, Mai LP, Tan HH, Lin QX, Yang M, Lin SG (2012) Integrating interacting drugs and genetic variations to improve the predictability of warfarin maintenance dose in Chinese patients. Pharmacogenet Genomics. doi:10.1097/FPC.0b013e32834f45f9
Soares RA, Santos PC, Machado-Coelho GL, Nascimento RM, Mill JG, Krieger JE, Pereira AC (2010) CYP2C9 and VKORC1 polymorphisms are differently distributed in the Brazilian population according to self-declared ethnicity or genetic ancestry. Genet Test Mol Biomarkers 16(8):957–963. doi:10.1089/gtmb.2012.0019
Introcaso G, Gesu G (2004) Significance of consecutive international normalized ratio (INR) outcomes using statistical control rules in long-term anticoagulated patients. Optimization of laboratory monitoring and interpretation of borderline measurements. Clin Chem Lab Med 42(3):294–299. doi:10.1515/CCLM.2004.053
Lassen JF, Kjeldsen J, Antonsen S, Hyltoft Petersen P, Brandslund I (1995) Interpretation of serial measurements of international normalized ratio for prothrombin times in monitoring oral anticoagulant therapy. Clin Chem 41(8 Pt 1):1171–1176
van den Besselaar AM, Fogar P, Pengo V, Palareti G, Braham S, Moia M, Tripodi A (2012) Biological variation of INR in stable patients on long-term anticoagulation with warfarin. Thromb Res 130(3):535–537. doi:10.1016/j.thromres.2012.05.028
Ward LS (2010) The difficult patient: drug interaction and the influence of concomitant diseases on the treatment of hypothyroidism. Arq Bras Endocrinol Metabol 54(5):435–442
Santos PC, Alvim Rde O, Ferreira NE, de Sa CR, Krieger JE, Mill JG, Pereira AC (2011) Ethnicity and arterial stiffness in Brazil. Am J Hypertens 24(3):278–284. doi:10.1038/ajh.2010.244
Acknowledgments
PCJLS and RAGS are recipients of fellowships from FAPESP, Brazil, Proc.2010-17465-8 and Proc.2010-17881-1. Technical assistance from the Laboratory of Genetics and Molecular Cardiology group, Heart Institute group is gratefully acknowledged.
Conflict of interests
The authors declare that they have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
Primers used for amplification of the CYP2C9 and VKORC1 polymorphisms and corresponding annealing temperatures (DOC 33 kb)
Rights and permissions
About this article
Cite this article
Santos, P.C.J.L., Dinardo, C.L., Schettert, I.T. et al. CYP2C9 and VKORC1 polymorphisms influence warfarin dose variability in patients on long-term anticoagulation. Eur J Clin Pharmacol 69, 789–797 (2013). https://doi.org/10.1007/s00228-012-1404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1404-5