Abstract
Purpose
The objective of this study was to compare different methods of adjusted indirect comparisons that can be used to investigate the relative bioavailability of different generic products. To achieve this goal, generic artemether/lumefantrine 20/120 mg tablets that have been prequalified by the World Health Organization (WHO) were selected as model products for study.
Methods
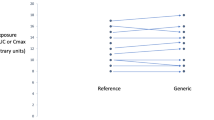
Data from three bioequivalence studies conducted independently that compared three generics with the same reference product were used to indirectly determine the relative bioavailability between the generics themselves.
Results
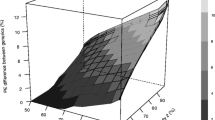
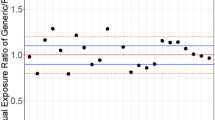
The different methods of indirect comparison examined in this study provide consistent results. Methods based on the assumption of a large sample size give slightly narrower 90 % confidence intervals. Therefore, the use of methods based on the t test is recommended. Given the precision of the area under the time–concentration curve (AUC) data, it is possible to conclude that the extent of exposure of artemether and lumefantrine is bioequivalent between the different generics studied. However, given the precision of the drug peak concentration (Cmax) data, it is not possible to demonstrate equivalence within the conventional acceptance range for all comparisons; it is possible to conclude bioequivalence within the widened acceptance range 75–133 %.
Conclusions
From a clinical viewpoint, not only are these prequalified generics bioequivalent and interchangeable with the reference product (Coartem, Novartis), but also the existing indirect evidence makes it possible to conclude that these WHO prequalified products are bioequivalent between themselves with respect to the AUC. The lack of the necessary precision to demonstrate bioequivalence between generics with respect to the Cmax within the conventional acceptance range does not preclude considering them as interchangeable, if necessary, since Cmax is considered to be of less clinical relevance for the relevant therapy.


Similar content being viewed by others
Notes
The WHO Prequalification of Medicines Programme was launched in 2001, in partnership with UNAIDS, UNICEF, and UNFPA, with support from the World Bank. Its original focus was the evaluation of medicines for treatment of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), malaria, and tuberculosis. Products in other therapeutic categories are now also assessed. The Programme reviews product dossiers, mainly for generic products, according to stringent, internationally accepted criteria, including data on product quality and bioequivalence, and inspects the corresponding manufacturing sites to assess compliance with good manufacturing practices (GMP). It also inspects contract research organizations (CROs) to verify compliance of the bioequivalence studies with good clinical practice (GCP) and good laboratory practices (GLP).
References
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) (2003) Guidance for Industry. Bioavailability and bioequivalence. Studies for orally adminsitered drug products—general considerations. CDER, Washington D.C.
Committee for Medicinal Products for Human Use (CHMP) (2010) Guideline on the investigation of bioequivalence. European Medicines Agency (EMA), London
WHO Expert Committee on Specifications for Pharmaceutical Preparations, World Health Organization (2006) Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability. WHO Technical Report Series 937. . WHO Press, Geneva, pp 347–390
Chow SC, Liu J (1997) Meta-analysis for bioequivalence review. J Biopharm Stat 7(1):97–111
Chow SC, Shao J (1999) Bioequivalence review for drug interchangeability. J Biopharm Stat 9(3):485–497
Bucher HC, Guyatt GH, Griffith LE, Walter SD (1997) The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 50(6):683–691
Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, Bradburn M, Eastwood AJ (2005) Indirect comparisons of competing interventions. Health Technol Assess 9(26):1–148
Maliepaard M, Banishki N, Gispen-de Wied CC, Teerenstra S, Elferink AJ (2011) Interchangeability of generic anti-epileptic drugs: a quantitative analysis of topiramate and gabapentin. Eur J Clin Pharmacol 67:1007–1016
Krauss GL, Caffo B, Chang YT, Hendrix CW, Chuang K (2011) Assessing bioequivalence of generic antiepilepsy drugs. Ann Neurol 70(2):221–228
Welch BL (1947) The generalisation of student’s problems when several different population variances are involved. Biometrika 34(1–2):28–35
Schottker B, Luhmann D, Boulkhemair D, Raspe H (2009) Indirect comparisons of therapeutic interventions. GMS Health Technol Assess 5:Doc09
Johnston A, He X, Holt DW (2006) Bioequivalence of enteric-coated mycophenolate sodium and mycophenolate mofetil: a meta-analysis of three studies in stable renal transplant recipients. Transplantation 82(11):1413–1418
Song F, Altman DG, Glenny AM, Deeks JJ (2003) Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. Br Med J 326(7387):472
Privitera M (2011) Is antiepileptic drug generic substitution always safe? Slow progress toward definitive answers. Ann Neurol 70(2):192–193
Edwards SJ, Clarke MJ, Wordsworth S, Borrill J (2009) Indirect comparisons of treatments based on systematic reviews of randomised controlled trials. Int J Clin Pract 63(6):841–854
Song F, Harvey I, Lilford R (2008) Adjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. J Clin Epidemiol 61(5):455–463
García Arieta A, Blázquez Pérez A, Rodriguez Mendizabal M (2006) Interchangeability between the different generic products in Spain: Gabapentin. In: 20th Congreso Nacional de la Sociedad Española de Farmacología Clínica, Puerto de la Cruz
Almeida Lopes R, Rocha Neves FA (2010) Meta-analysis for bioequivalence studies: interchangeability of generic drugs and similar containing Hydrochlorothiazide is possible but not for those with Enalapril Maleate. J Bras Nefrol 32(2):173–181
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript represents the personal opinion of the authors and does not necessarily represent the views or policy of their corresponding Regulatory Agencies or the World Health Organization
Rights and permissions
About this article
Cite this article
Gwaza, L., Gordon, J., Welink, J. et al. Statistical approaches to indirectly compare bioequivalence between generics: a comparison of methodologies employing artemether/lumefantrine 20/120 mg tablets as prequalified by WHO. Eur J Clin Pharmacol 68, 1611–1618 (2012). https://doi.org/10.1007/s00228-012-1396-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1396-1