Abstract
Rationale
Buspirone, a partial 5HT1A agonist and D2 and D3 antagonist, has shown promising antiemetic efficacy when given parenterally in animal models, but its efficacy for the prevention of postoperative nausea and vomiting (PONV) is unknown.
Objective
To study the efficacy and dose-responsiveness of intravenous buspirone for the prevention of PONV.
Methods
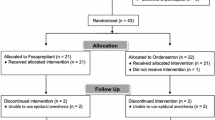
A randomised, double-blind, placebo-controlled study was performed in adults at moderate to high PONV risk undergoing surgery with a general anaesthetic. Patients were randomised to receive an intravenous dose of buspirone (0.3, 1.0, 2.0, 3.0 mg) or placebo at the end of surgery. The primary endpoint was the cumulative 24-h PONV incidence (i.e. any nausea and/or vomiting). Vomiting included retching. Nausea was defined as a score of ≥4 on an 11-point verbal rating scale running from zero (no nausea) to ten (the worst nausea imaginable).
Results
A total of 257 patients received the study drug and fulfilled the criteria for inclusion in the primary efficacy and safety analyses. With placebo, the mean 24-h PONV incidence was 49.0 % (90 % confidence interval [CI] 37.5–60.5 %). With buspirone, that incidence ranged from a mean of 40.8 % (29.3–52.4 %) in the 1 mg arm to 58.0 % (46.5–69.5 %) in the 0.3 mg arm (P > 0.05 for all comparisons). There was no difference between placebo and buspirone at any dose for any other efficacy endpoint, nor in the number or severity of adverse events or any other safety measures.
Conclusion
We were unable to show that intravenous single-dose buspirone, at the tested dose-range, was effective at preventing PONV in surgical adult patients. The present study emphasises the difficulty in extrapolating from animal models of emesis to clinical efficacy in PONV.


Similar content being viewed by others
Abbreviations
- D:
-
Dopaminergic
- 5HT:
-
Serotoninergic
- NK:
-
Neurokinin
- PONV:
-
Postoperative nausea and vomiting
- PP:
-
Pyrimidinylpiperazine
References
Kranke P (2011) Effective management of postoperative nausea and vomiting: let us practise what we preach! Eur J Anaesthesiol 28:152–154
Pierre S (2011) Risk scores for predicting postoperative nausea and vomiting are clinically useful tools and should be used in every patient: pro–'don't throw the baby out with the bathwater'. Eur J Anaesthesiol 28:160–163
Sanger GJ, Andrews PL (2006) Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci 129:3–16
Oshima T, Kasuya Y, Okumura Y, Terazawa E, Dohi S (2002) Prevention of nausea and vomiting with tandospirone in adults after tympanoplasty. Anesth Analg 95:1442–1445
Darmani NA, Zhao W, Ahmad B (1999) The role of D2 and D3 dopamine receptors in the mediation of emesis in Cryptotis parva (the least shrew). J Neural Transm 106:1045–1061
Kranke P, Eberhart LH (2011) Possibilities and limitations in the pharmacological management of postoperative nausea and vomiting. Eur J Anaesthesiol 28:758–765
Kooij FO, Klok T, Hollmann MW, Kal JE (2010) Automated reminders increase adherence to guidelines for administration of prophylaxis for postoperative nausea and vomiting. Eur J Anaesthesiol 27:187–191
Habib AS, Gan TJ (2005) The effectiveness of rescue antiemetics after failure of prophylaxis with ondansetron or droperidol: a preliminary report. J Clin Anesth 17:62–65
Tramèr MR (2003) Treatment of postoperative nausea and vomiting. Br Med J 327:762–763
Riblet LA, Taylor DP, Eison MS, Stanton HC (1982) Pharmacology and neurochemistry of buspirone. J Clin Psychiatry 43:11–18
Gupta YK, Sharma SS (2002) Involvement of 5-HT1A and 5-HT2 receptor in cisplatin induced emesis in dogs. Indian J Physiol Pharmacol 46:463–467
Lucot JB, Crampton GH (1987) Buspirone blocks cisplatin-induced emesis in cats. J Clin Pharmacol 27:817–818
Lucot JB, Crampton GH (1987) Buspirone blocks motion sickness and xylazine-induced emesis in the cat. Aviat Space Environ Med 58:989–991
Okada F, Torii Y, Saito H, Matsuki N (1994) Antiemetic effects of serotonergic 5-HT1A-receptor agonists in Suncus murinus. Jpn J Pharmacol 64:109–114
Alfieri AB, Cubeddu LX (1995) Comparative efficacy of a single oral dose of ondansetron and of buspirone against cisplatin-induced emesis in cancer patients. Br J Cancer 72:1013–1015
Gammans RE, Mayol RF, LaBudde JA (1986) Metabolism and disposition of buspirone. Am J Med 80:41–51
Center for Drug Evaluation and Research (2005) Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. United States Food and Drug Administration, Rockville
Aldrete JA (1995) The post-anesthesia recovery score revisited. J Clin Anesth 7:89–91
Peroutka SJ (1985) Selective interaction of novel anxiolytics with 5-hydroxytryptamine1A receptors. Biol Psychiatry 20:971–979
Romero AG, Leiby JA, McCall RB, Piercey MF, Smith MW, Han F (1993) Novel 2-substituted tetrahydro-3 H-benz.eindolamines: highly potent and selective agonists acting at the 5-HT1A receptor as possible anxiolytics and antidepressants. J Med Chem 36:2066–2074
Newman-Tancredi A, Gavaudan S, Conte C, Chaput C, Touzard M, Verrièle L, Audinot V, Millan MJ (1998) Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a.35SGTPgammaS binding study. Eur J Pharmacol 355:245–256
Yocca FD (1990) Neurochemistry and neurophysiology of buspirone and gepirone: interactions at presynaptic and postsynaptic 5-HT1A receptors. J Clin Psychopharmacol 10:6S–12S
Ministry of Health Labour and Welfare (Japan) (1997) Summary basis of approval, tandospirone citrate tablets. Ministry of Health, Labour and Welfare, Tokyo
Díaz-Mataix L, Scorza MC, Bortolozzi A, Toth M, Celada P, Artigas F (2005) Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci 25:10831–10843
Zhu BT (2005) Mechanistic explanation for the unique pharmacologic properties of receptor partial agonists. Biomed Pharmacother 59:76–89
Seletti B, Benkelfat C, Blier P, Annable L, Gilbert F, de Montigny C (1995) Serotonin1A receptor activation by flesinoxan in humans. Body temperature and neuroendocrine responses. Neuropsychopharmacology 13:93–104
Grof P, Joffe R, Kennedy S, Persad E, Syrotiuk J, Bradford D (1993) An open study of oral flesinoxan, a 5-HT1A receptor agonist, in treatment-resistant depression. Int Clin Psychopharmacol 8:167–172
Lutsep HL (2005) Repinotan, A 5-HT1A agonist, in the treatment of acute ischemic stroke. Curr Drug Targets CNS Neurol Disord 4:119–120
Lucot JB (1994) Antiemetic effects of flesinoxan in cats: comparisons with 8-hydroxy-2-(di-n-propylamino)tetralin. Eur J Pharmacol 253:53–60
Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekström JC, Svenningsson P, Meister B, Kehr J, Stiedl O (2008) The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res 195:54–77
Acknowledgements
The study was funded by Acacia Pharma Ltd, the manufacturer of the study drug. The study design was developed by a steering committee (CCA, PD, TJG, PK, MRT) in collaboration with Acacia Pharma Ltd. Acacia Pharma Ltd edited the study protocol in collaboration with the steering committee members, organised meetings for steering committee members and investigators, supplied study drugs and was responsible for data analysis.
Participating study investigators and centres
France: Prof Hervé Bouaziz, Hôpital Central, Nancy; Prof Dominique Chassard, Hôpital Mere Enfant, Bron; Prof Pierre Diemunsch, CHU de Hautepierre, Strasbourg; Prof Gilles Lebuffe, Hôpital Huriez–CHRU, Lille; Dr Ngay Liu, Hôpital Foch, Suresnes; Prof Jean-Marc Malinowsky, Hôpital Maison Blanche–CHRU, Reims; Prof Emmanuel Samain, CHU Besançon, Besançon. Germany: Prof Leopold Eberhart, Philipps Universität Marburg, Marburg; Prof Peter Kranke and Dr Mustafa Rifai, Universitätsklinik Würzburg, Würzburg; Prof Johann Motsch, Universität Heidelberg, Heidelberg; Dr Kerstin D Röhm, Klinikum Ludwigshafen, Ludwigshafen; Prof Claudia Spies and Dr Martin Franck, Charité–Universitätsmedizin Berlin, Berlin; Dr Jan Wallenborn, Universität Leipzig, Leipzig. Switzerland: Prof Martin Tramèr and Dr Georges Savoldelli, Geneva University Hospitals, Geneva. USA: Prof Christian Apfel, UCSF Medical Center at Mt Zion, San Francisco, CA; Prof Tong Joo Gan and Dr Ashraf S Habib, Duke University Medical Center, Durham, NC; Dr Harold S Minkowitz, Memorial Hermann-Memorial City Hospital, Houston, TX
Conflict of interest and disclosure
There was a pre hoc agreement that the investigators would have the right to examine the data independently and to submit a manuscript for publication without first obtaining the consent of the sponsor and that the results of this study would be published as a full report, in their entirety and not as individual centre data, and independent of the result of the study. MRT had full access to all the data and coordinated the decision to submit for publication. All authors participated in writing the manuscript. GMF is an employee of Acacia Pharma Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration
Clinicaltrials.gov identifier: NCT00895830.
Rights and permissions
About this article
Cite this article
Kranke, P., Röhm, K.D., Diemunsch, P. et al. Intravenous buspirone for the prevention of postoperative nausea and vomiting. Eur J Clin Pharmacol 68, 1465–1472 (2012). https://doi.org/10.1007/s00228-012-1284-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1284-8