Abstract
Purpose
The influence of the cytochrome P450 enzyme CYP2D6 in the metabolism of the novel dopaminergic stabilizer pridopidine was investigated in healthy Swedish Caucasians.
Methods
Six extensive metabolizers (EM) and six poor metabolizers (PM) of debrisoquine were given a single oral dose of pridopidine (EM, 50 mg; PM, 25 mg).
Results
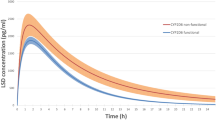
The mean total plasma clearance of pridopidine was 541 and 138 mL/min in EM and PM, respectively (p = 0.003), and was slightly higher in PM than the mean renal plasma clearance (105 mL/min; p = 0.11). The mean plasma area under the time–concentration curve between time zero and 32 h (AUC0-32h) of the N-depropyl metabolite ACR30 was higher in EM than in PM (1,377 vs. 61 nmol h/mL, respectively; p < 0.001). The urinary excretion of pridopidine + ACR30 was high in both EM (85 %) and PM (78 %). The dose-adjusted peak concentration (Cmax) was not statistically different in EM and PM; consequently, the oral absorption of pridopidine was close to complete.
Conclusions
Following a single dose of pridopidine, the drug is N-depropylated by CYP2D6 in EM, while in PM the most important elimination pathway is renal glomerular filtration. Results of studies examining the effects of multiple repeat dosing suggest that the CYP2D6 enzyme is at least partly inactivated by pridopidine.



Similar content being viewed by others
References
Bertilsson L (2007) Metabolism of antidepressant and neuroleptic drugs by cytochrome P450s: clinical and interethnic aspects. Clin Pharmacol Ther 82:606–609
Dahl M, Johansson I, Bertilsson L, Ingelman-Sundberg M, Sjoqvist F (1995) Ultrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basis. J Pharmacol Exp Ther 274:516–520
Aklillu E, Persson I, Bertilsson L, Johansson I, Rodrigues F, Ingelman-Sundberg M (1996) Frequent distribution of ultrarapid metabolizers of debrisoquine in an ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J Pharmacol Exp Ther 278:441–446
Dyhring T, Nielsen EØ, Sonesson C, Pettersson F, Karlsson J, Svensson P, Christophersen P, Waters N (2010) The dopaminergic stabilizers pridopidine (ACR16) and (−)-OSU6162 display dopamine D(2) receptor antagonism and fast receptor dissociation properties. Eur J Pharmacol 628:19–26
Pontén H, Kullingsjö J, Lagerkvist S, Martin P, Pettersson F, Sonesson C, Waters S, Waters N (2010) In vivo pharmacology of the dopaminergic stabilizer pridopidine. Eur J Pharmacol 644:88–95
Waters N, Carlsson A, Carlsson M, Martin P, Tedroff J, Kullingsjo J, Petterson F, Sonesson C, Waters E (2002) Pharmacology of ACR16, a dopamine stabilizer. Oral presentation; Program No. 894.13. 2002 Neuroscience Meeting Planner. Orlando, FL: Society for Neuroscience, 2002. Online.
Natesan S, Svensson KA, Reckless GE, Nobrega JN, Barlow KB, Johansson AM, Kapur S (2006) The dopamine stabilizers (S)-(−)-(3-methanesulfonyl-phenyl)-1-propyl-piperidine [(−)-OSU6162] and 4-(3-methanesulfonylphenyl)-1-propyl-piperidine (ACR16) show high in vivo D2 receptor occupancy, antipsychotic-like efficacy, and low potential for motor side effects in the rat. J Pharmacol Exp Ther 318:810–818
Rung JP, Rung E, Helgeson L, Johansson AM, Svensson K, Carlsson A, Carlsson ML (2008) Effects of (−)-OSU6162 and ACR16 on motor activity in rats, indicating a unique mechanism of dopaminergic stabilization. J Neural Transm 115:899–908
Pettersson F, Pontén H, Waters N, Waters S, Sonesson C (2010) Synthesis and evaluation of a set of 4-phenylpiperidines and 4-phenylpiperazines as D(2) receptor ligands and the discovery of the dopaminergic stabilizer 4-[3-(methylsulfonyl)phenyl]-1-propylpiperidine (Huntexil, Pridopidine, ACR16). J Med Chem 53:2510–2520
Carlsson ML, Carlsson A, Nilsson M (2004) Schizophrenia: from dopamine to glutamate and back. Curr Med Chem 11:267–277
Lundin A, Dietrichs E, Haghighi S, Göller ML, Heiberg A, Loutfi G, Widner H, Wiktorin K, Wiklund L, Svenningsson A, Sonesson C, Waters N, Waters S, Tedroff J (2010) Efficacy and safety of the dopaminergic stabilizer Pridopidine (ACR16) in patients with Huntington's disease. Clin Neuropharmacol 33:260–264
de Yebenes JG, Landwehrmeyer B, Squitieri F, Reilmann R, Rosser A, Barker RA, Saft C, Magnet MK, Sword A, Rembratt A, Tedroff J (2011) Pridopidine for the treatment of motor function in patients with Huntington's disease (MermaiHD): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol 10:1049–1057
Kieburtz K, on behalf of the HSG HART study investigators (2011) A randomized, double-blind, placebo-controlled trial of ACR16 in Huntington's disease. Neurotherapeutics 8:135
Landwehrmeyer B, Marder K, Biilmann Rønn B, Haglund M, MermaiHD, on behalf of the HSG HART study investigators (2011) Effect of the dopaminergic stabilizer pridopidine on motor symptoms in Huntington’s disease: a meta-analysis (abstract 211). Poster session presented at World Congress on Huntington’s Disease; 2011 Sept 11–14; Melbourne, Australia. Clin Genet 80
Acknowledgements
This study was sponsored by grants from NeuroSearch Sweden AB and from the Swedish Research Council, Medicine (3902). EU Orphan designation number EU/3/05/288.
Conflict of interest/disclosure
N. Waters, S. Waters and J. Tedroff are employed at NeuroSearch Sweden AB, which sponsors the clinical development of pridopidine. The remaining authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Helldén, A., Panagiotidis, G., Johansson, P. et al. The dopaminergic stabilizer pridopidine is to a major extent N-depropylated by CYP2D6 in humans. Eur J Clin Pharmacol 68, 1281–1286 (2012). https://doi.org/10.1007/s00228-012-1248-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1248-z