Abstract
Objective
Our objective was to describe the time course of the placebo effect in asthma and quantitatively investigate the affective factors of the placebo effect for the placebo response simulation during the asthma clinical study design.
Methods
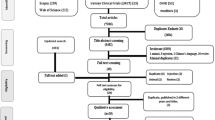
We conducted a systemic search of public data sources for the study-level forced expiratory volume in 1 second (FEV1) to build the placebo effect model for studies by oral or inhaled administrations simultaneously. The administration routes, types of inhalation device, mean patient age, mean male proportion, baseline FEV1, disease severity, year of publication, inhaled corticosteroid status during the treatment, and dropout rate were tested as covariates.
Results
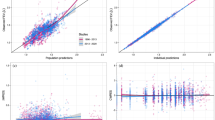
There are 34 literature sources containing 178 mean values for FEV1 presenting the individual observations from about 3,703 patients. The exponential models adequately described the time course of placebo effect with the typical value of the maximum placebo effect (Pmax) of 0.060 L. Dropout rate incorporated in the residual error model and the disease severity (mild to moderate and moderate to severe) at baseline were covariates that remained in the final model.
Conclusions
The placebo effect is adequately described by an exponential model over time. By incorporating the dropout rate in the residual error model, the estimation precision was improved. The model could predict the placebo response profile in mild to severe asthmatic patients for the asthma clinical study design and could also be a structure model of the placebo effect for the pure drug effect evaluation in the asthma clinical trials.



Similar content being viewed by others
References
Matre D, Casey KL, Knardahl S (2006) Placebo-induced changes in spinal cord pain processing. J Neurosci 26(2):559–563. doi:10.1523/JNEUROSCI.4218-05.2006
Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA (2002) The functional neuroanatomy of the placebo effect. Am J Psychiatry 159(5):728–737
de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ (2001) Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 293(5532):1164–1166. doi:10.1126/science.1060937
Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN (2007) Placebo response in asthma: a robust and objective phenomenon. J Allergy Clin Immunol 119(6):1375–1381. doi:10.1016/j.jaci.2007.03.016
Joyce DP, Jackevicius C, Chapman KR, McIvor RA, Kesten S (2000) The placebo effect in asthma drug therapy trials: a meta-analysis. J Asthma 37(4):303–318
Delahoy P, Thompson S, Marschner IC (2010) Pregabalin versus gabapentin in partial epilepsy: a meta-analysis of dose–response relationships. BMC Neurol 10:104. doi:10.1186/1471-2377-10-104
Houk BE, Bello CL, Kang D, Amantea M (2009) A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 15(7):2497–2506. doi:10.1158/1078-0432.ccr-08-1893
Chan PL, Weatherley B, McFadyen L (2008) A population pharmacokinetic meta-analysis of maraviroc in healthy volunteers and asymptomatic HIV-infected subjects. Br J Clin Pharmacol 65(Suppl 1):76–85. doi:10.1111/j.1365-2125.2008.03139.x
Ito K, Ahadieh S, Corrigan B, French J, Fullerton T, Tensfeldt T (2010) Disease progression meta-analysis model in Alzheimer's disease. Alzheimers Dement 6(1):39–53. doi:10.1016/j.jalz.2009.05.665
Leroyer C, Perfetti L, Trudeau C, L'ARCHEVQUE J, Chan-Yeung M, Malo JL (1998) Comparison of serial monitoring of peak expiratory flow and FEV1 in the diagnosis of occupational asthma. Am J Respir Crit Care Med 158(3):827
Wechsler ME, Kelley JM, Boyd IOE, Dutile S, Marigowda G, Kirsch I, Israel E, Kaptchuk TJ (2011) Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med 365(2):119–126. doi:10.1056/NEJMoa1103319
Dansirikul C, Silber HE, Karlsson MO (2008) Approaches to handling pharmacodynamic baseline responses. J Pharmacokinet Pharmacodyn 35(3):269–283. doi:10.1007/s10928-008-9088-2
Team R (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna Austria. doi:http://www.R-project.org
Jonsson EN, Karlsson MO (1998) Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58(1):51–64
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75(2):85–94. doi:10.1016/j.cmpb.2003.11.003
Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD (2011) Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed 101(1):72–79. doi:10.1016/j.cmpb.2010.04.018
Israel E, Rubin P, Kemp JP, Grossman J, Pierson W, Siegel SC, Tinkelman D, Murray JJ, Busse W, Segal AT, Fish J, Kaiser HB, Ledford D, Wenzel S, Rosenthal R, Cohn J, Lanni C, Pearlman H, Karahalios P, Drazen JM (1993) The effect of inhibition of 5-lipoxygenase by zileuton in mild-to-moderate asthma. Ann Intern Med 119(11):1059–1066
Persson G, Baas A, Knight A, Larsen B, Olsson H (1995) One month treatment with the once daily oral (beta)2-agonist bambuterol in asthmatic patients. Eur Respir J 8(1):34–39. doi:10.1183/09031936.95.08010034
Israel E, Cohn J, Dube L, Drazen JM (1996) Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. a randomized controlled trial. Zileuton clinical trial group. JAMA 275(12):931–936. doi:10.1001/jama.1996.03530360041036
Liu MC, Dube LM, Lancaster J (1996) Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: a 6-month randomized multicenter trial. Zileuton study group. J Allergy Clin Immunol 98(5 Pt 1):859–871. doi:10.1016/S0091-6749(96)80002-9
Barnes NC, Pujet JC (1997) Pranlukast, a novel leukotriene receptor antagonist: results of the first European, placebo controlled, multicentre clinical study in asthma. Thorax 52(6):523–527. doi:10.1136/thx.52.6.523
Grossman J, Faiferman I, Dubb JW, Tompson DJ, Busse W, Bronsky E, Montanaro A, Southern L, Tinkelman D (1997) Results of the first U.S. double-blind, placebo-controlled, multicenter clinical study in asthma with pranlukast, a novel leukotriene receptor antagonist. J Asthma 34(4):321–328
Altman LC, Munk Z, Seltzer J, Noonan N, Shingo S, Zhang J, Reiss TF (1998) A placebo-controlled, dose-ranging study of montelukast, a cysteinyl leukotriene-receptor antagonist. Montelukast asthma study group. J Allergy Clin Immunol 102(1):50–56. doi:10.1016/S0091-6749(98)70054-5
Nathan RA, Bernstein JA, Bielory L, Bonuccelli CM, Calhoun WJ, Galant SP, Hanby LA, Kemp JP, Kylstra JW, Nayak AS, O'Connor JP, Schwartz HJ, Southern DL, Spector SL, Williams PV (1998) Zafirlukast improves asthma symptoms and quality of life in patients with moderate reversible airflow obstruction. J Allergy Clin Immunol 102(6 Pt 1):935–942. doi:10.1016/S0091-6749(98)70331-8
Noonan MJ, Chervinsky P, Brandon M, Zhang J, Kundu S, McBurney J, Reiss TF (1998) Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Asthma Study Group. Montelukast Eur Respir J 11(6):1232–1239. doi:10.1183/09031936.98.11061232
Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB (1998) Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: A multicenter, randomized, double-blind trial. Arch Intern Med 158(11):1213–1220
Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF (1999) Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/beclomethasone study group. Ann Intern Med 130(6):487–495. doi:199903160-00005
Yoo SH, Park SH, Song JS, Kang KH, Park CS, Yoo JH, Choi BW, Hahn MH (2001) Clinical effects of pranlukast, an oral leukotriene receptor antagonist, in mild-to-moderate asthma: a 4week randomized multicentre controlled trial. Respirology 6(1):15–21
Nelson H, Kemp J, Berger W, Corren J, Casale T, Dube L, Walton-Bowen K, LaVallee N, Stepanians M (2007) Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol 99(2):178–184. doi:10.1016/S1081-1206(10)60642-4
Lu S, Liu N, Dass SB, Reiss TF, Knorr BA (2009) Randomized, placebo-controlled study of a selective PDE4 inhibitor in the treatment of asthma. Respir Med 103(3):342–347. doi:10.1016/j.rmed.2008.10.024
Physicians ACoC (1990) A double-blind multicenter group comparative study of the efficacy and safety of nedocromil sodium in the management of asthma. North American tilade study group. Chest 97(6):1299–1306. doi:10.1378/chest.97.6.1299
Pearlman DS, Berger WE, Kerwin E, Laforce C, Kundu S, Banerji D (2005) Once-daily ciclesonide improves lung function and is well tolerated by patients with mild-to-moderate persistent asthma. J Allergy Clin Immunol 116(6):1206–1212. doi:10.1016/j.jaci.2005.08.037
Chuchalin AG, Tsoi AN, Richter K, Krug N, Dahl R, Luursema PB, Cameron R, Bao W, Higgins M, Woessner R, van As A (2007) Safety and tolerability of indacaterol in asthma: a randomized, placebo-controlled 28-day study. Respir Med 101(10):2065–2075. doi:10.1016/j.rmed.2007.06.002
Fernandes AL, Faresin SM, Amorim MM, Fritscher CC, Pereira CA, Jardim JR (2001) Inhaled budesonide for adults with mild-to-moderate asthma: a randomized placebo-controlled, double-blind clinical trial. Sao Paulo Med J 119(5):169–174. doi:10.1590/S1516-31802001000500004
Yang WH, Martinot JB, Pohunek P, Beier J, Magula D, Cameron R, Owen R, Higgins M (2007) Tolerability of indacaterol, a novel once-daily beta2-agonist, in patients with asthma: a randomized, placebo-controlled, 28-day safety study. Ann Allergy Asthma Immunol 99(6):555–561. doi:10.1016/S1081-1206(10)60386-9
Nayak AS, Banov C, Corren J, Feinstein BK, Floreani A, Friedman BF, Goldsobel A, Gottschlich GM, Hannaway PJ, Lampl KL, Lapidus RJ, Lawrence M, Lumry W, Munk Z, Pearlman D, Scardella AT, Schenkel EJ, Segal AT, Segall N, Silverman B, Shneyer L, Nolop KB, Harrison JE (2000) Once-daily mometasone furoate dry powder inhaler in the treatment of patients with persistent asthma. Ann Allergy Asthma Immunol 84(4):417–424. doi:10.1016/s1081-1206(10)62275-2
Kemp JP, Berkowitz RB, Miller SD, Murray JJ, Nolop K, Harrison JE (2000) Mometasone furoate administered once daily is as effective as twice-daily administration for treatment of mild-to-moderate persistent asthma. J Allergy Clin Immunol 106(3):485–492. doi:10.1067/mai.2000.109431
Bronsky E, Korenblat P, Harris AG, Chen R (1998) Comparative clinical study of inhaled beclomethasone dipropionate and triamcinolone acetonide in persistent asthma. Ann Allergy Asthma Immunol 80(4):295–302. doi:10.1016/S1081-1206(10)62972-9
Ramsdell JW, Fish L, Graft D, Higgins N, Kavuru M, Pleskow W, Banerji D (1998) A controlled trial of twice daily triamcinolone oral inhaler in patients with mild-to-moderate asthma. Ann Allergy Asthma Immunol 80(5):385–390. doi:10.1016/S1081-1206(10)62988-2
Ohta K, Miyamoto T, Amagasaki T, Yamamoto M (2009) Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology 14(8):1156–1165. doi:10.1111/j.1440-1843.2009.01633.x
ZuWallack R, Adelglass J, Clifford DP, Duke SP, Wire PD, Faris M, Harding SM (2000) Long-term efficacy and safety of fluticasone propionate powder administered once or twice daily via inhaler to patients with moderate asthma. Chest 118(2):303–312
Selroos O (2008) Effect of disease duration on dose–response of inhaled budesonide in asthma. Respir Med 102(7):1065–1072. doi:10.1016/j.rmed.2007.12.029
Banov CH, Howland WC, Lumry WR (2001) Once-daily budesonide via Turbuhaler improves symptoms in adults with persistent asthma. Ann Allergy Asthma Immunol 86(6):627–632
Nathan RA, Li JTC, Finn A, Jones R, Payne JE, Wolford JP, Harding SM (2000) A dose-ranging study of fluticasone propionate administered once daily via multidose powder inhaler to patients with moderate asthma. Chest 118(2):296–302
Meltzer EO, Korenblat PE, Weinstein SF, Noonan M, Karafilidis J (2009) Efficacy and safety evaluation of ciclesonide in mild-tomoderate persistent asthma previously treated with inhaled corticosteroids. Allergy Asthma Proc 30(3):293–303. doi:10.2500/aap.2009.30.3241
McFadden ER, Casale TB, Edwards TB, Kemp JP, Metzger WJ, Nelson HS, Storms WW, Neidl MJ (1999) Administration of budesonide once daily by means of turbuhaler to subjects with stable asthma. J Allergy Clin Immunol 104(1):46–52
Berger WE, Kerwin E, Bernstein DI, Pedinoff A, Bensch G, Karafilidis J (2009) Efficacy and safety evaluation of ciclesonide in subjects with mild-to-moderate asthma not currently using inhaled corticosteroids. Allergy Asthma Proc 30(3):304–314. doi:10.2500/aap.2009.30.3242
Kerwin EM, Pearlman DS, De Guia T, Carlsson LG, Gillen M, Uryniak T, Simonson SG (2008) Evaluation of efficacy and safety of budesonide delivered via two dry powder inhalers. Curr Med Res Opin 24(5):1497–1510
Bernstein DI, Cohen R, Gichansky E, Pedinoff AJ, Tinkelman DG, Winder JA (1998) A multicenter, placebo-controlled study of twice daily triamcinolone acetonide (800 (mu)g per day) for the treatment of patients with mild-to- moderate asthma. J Allergy Clin Immunol 101(4 I):433–438
Ekstrom T, Ringdal N, Tukiainen P, Runnerstrom E, Soliman S (1998) A 3-month comparison of formoterol with terbutaline via turbuhaler. A placebo-controlled study. Ann Allergy Asthma Immunol 81(3):225–230
Benedetti F, Amanzio M, Baldi S, Casadio C, Maggi G (1999) Inducing placebo respiratory depressant responses in humans via opioid receptors. Eur J Neurosci 11(2):625–631. doi:10.1046/j.1460-9568.1999.00465.x
Hughes J, Gabbay M, Funnell E, Dowrick C (2011) Exploratory review of placebo characteristics reported in randomised placebo controlled antidepressant drug trials. Pharmacopsychiatry. doi:10.1055/s-0031-1286260
Macedo A, Farré M, Baños JE (2006) A meta-analysis of the placebo response in acute migraine and how this response may be influenced by some of the characteristics of clinical trials. Eur J Clin Pharmacol 62(3):161–172. doi:10.1007/s00228-005-0088-5
Acknowledgements
The authors gratefully acknowledge support from Dr. Shanshan Bi (Peking University) and Dr. Guangli Ma [Pfizer (China) Research & Development Center]. This work was supported by the Ministry of Science and Technology of the People’s Republic of China (Peking University New Drug Research & Development Platform, grant number 2009ZX09301-010).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Feng Guo and Wei Lu contributed equally to the concept and design of this study and can be considered to be co-corresponding authors.
Rights and permissions
About this article
Cite this article
Wang, X., Shang, D., Ribbing, J. et al. Placebo effect model in asthma clinical studies: longitudinal meta-analysis of forced expiratory volume in 1 second. Eur J Clin Pharmacol 68, 1157–1166 (2012). https://doi.org/10.1007/s00228-012-1245-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1245-2