Abstract
Purpose
In France, early detection of adverse effects does not currently involve any automatic signal detection method. The present objective was to assess the feasibility and measure the potential benefit of the incorporation of an automatic signal detection tool (GPSpH0) in the French pharmacovigilance system.
Methods
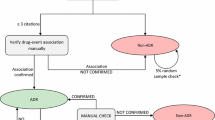
GPSpH0 was first applied to the data collected from 1 January 2000 to 31 December 2008 and then to the data collected from 1 January 2000 to 31 March 2009. A total of 1,414 original signals were detected. They were shared out for further expertise among 32 centres, i.e. the 31 Regional Pharmacovigilance Centres and the French medicine agency (AFSSAPS) pharmacovigilance department.
Results
The participating centres (n = 28) analysed 1,292 signals in May 2009. Overall, 277 signals whether known or unknown were thus considered worth following up. Half of the other 893 categorised signals were “well-known” (35.7%) and non-interpretable/non-pertinent signals (36.6%); 4% were not categorised because of a lack of time. Analysis of the signals was time-consuming, but the working time estimated by the participants was highly variable (median time: 6 h; minimum: 2 h maximum: 26 h).
Conclusions
The results of this study are in favour of the integration of an automated signal detection tool to complement the current pharmacovigilance activities. The Anatomic Therapeutic Chemical for drug classification poses difficulties in many situations; the international proprietary name might be more efficient. The variability observed in the time needed for analysis suggests that a standardised methodology should be employed. Overall, the findings of this prospective study will contribute to refining the signal management procedure to be implemented in the future.
Similar content being viewed by others
References
Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A et al (1998) Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 54(4):315–321
Norén GN, Bate A, Orre R, Edwards IR (2006) Extending the methods used to screen the WHO drug safety database towards analysis of complex associations and improved accuracy for rare events. Stat Med 25(21):3740–3757
DuMouchel W (1999) Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat 53(3):177–190
DuMouchel W, Pregibon D (2001) Empirical bayes screening for multi-item associations [Internet]. In: Proceedings of the Seventh ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. ACM, San Francisco, p 67–76. Available from: http://portal.acm.org/citation.cfm?id=502526. Accessed 26 March 2010
Szarfman A, Machado SG, O’Neill RT (2002) Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf 25(6):381–392
Van Puijenbroek EP, Bate A, Leufkens HGM, Lindquist M, Orre R, Egberts ACG (2002) A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 11(1):3–10
Evans SJ, Waller PC, Davis S (2001) Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 10(6):483–486
Hauben M, Madigan D, Gerrits CM, Walsh L, Van Puijenbroek EP (2005) The role of data mining in pharmacovigilance. Expert Opin Drug Saf 4(5):929–948
Bate A, Evans SJW (2009) Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 18(6):427–436
Roux E, Thiessard F, Fourrier A, Bégaud B, Tubert-Bitter P (2005) Evaluation of statistical association measures for the automatic signal generation in pharmacovigilance. IEEE Trans Inf Technol Biomed 9(4):518–527
Matsushita Y, Kuroda Y, Niwa S, Sonehara S, Hamada C, Yoshimura I (2007) Criteria revision and performance comparison of three methods of signal detection applied to the spontaneous reporting database of a pharmaceutical manufacturer. Drug Saf 30(8):715–726
Ahmed I, Thiessard F, Miremont-Salamé G, Bégaud B, Tubert-Bitter P (2010) Pharmacovigilance data mining with methods based on false discovery rates: a comparative simulation study. Clin Pharmacol Ther 88(4):492–498
Hochberg AM, Hauben M, Pearson RK, O’Hara DJ, Reisinger SJ, Goldsmith DI et al (2009) An evaluation of three signal-detection algorithms using a highly inclusive reference event database. Drug Saf 32(6):509–525
Thiessard F (2004) Détection des effets indésirables des médicaments par un système de génération automatisée du signal adapté à la base nationale de pharmacovigilance [thesis]. Université Victor Ségalen Bordeaux 2
Lehman HP, Chen J, Gould AL, Kassekert R, Beninger PR, Carney R et al (2007) An evaluation of computer-aided disproportionality analysis for post-marketing signal detection. Clin Pharmacol Ther 82(2):173–180
Hochberg AM, Hauben M, Pearson RK, O’Hara DJ, Reisinger SJ (2009) Systematic investigation of time windows for adverse event data mining for recently approved drugs. J Clin Pharmacol 49(6):626–633
Alvarez Y, Hidalgo A, Maignen F, Slattery J (2010) Validation of statistical signal detection procedures in eudravigilance post-authorization data: a retrospective evaluation of the potential for earlier signalling. Drug Saf 33(6):475–487
Ahmed I, Thiessard F, Miremont-Salamé G, Haramburu F, Kreft-Jais C, Bégaud B et al. Early detection of pharmacovigilance signals with automated methods based on false discovery rates: a comparative study. Drug Saf (in press)
Hochberg AM, Hauben M (2009) Time-to-signal comparison for drug safety data-mining algorithms vs. traditional signaling criteria. Clin Pharmacol Ther 85(6):600–606
Ahmed I, Haramburu F, Fourrier-Réglat A, Thiessard F, Kreft-Jais C, Miremont-Salamé G et al (2009) Bayesian pharmacovigilance signal detection methods revisited in a multiple comparison setting. Stat Med 28(13):1774–1792
Ahmed I, Dalmasso C, Haramburu F, Thiessard F, Broët P, Tubert-Bitter P (2010) False discovery rate estimation for frequentist pharmacovigilance signal detection methods. Biometrics 66(1):301–309
Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJW, Yuen N (2007) Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther 82(2):157–166
Thiessard F, Roux E, Miremont-Salamé G, Fourrier-Réglat A, Haramburu F, Tubert-Bitter P et al (2005) Trends in spontaneous adverse drug reaction reports to the French pharmacovigilance system (1986–2001). Drug Saf 28(8):731–740
R Development Core Team (2011) R: a language and environment for statistical computing [internet]. Vienna, Austria. Available from: http://www.R-project.org
Ahmed I, Poncet A (2010) PhViD: a R package for PharmacoVigilance signal detection [internet]. Available from: http://cran.r-project.org/web/packages/PhViD/index.html
Bégaud B, Evreux JC, Jouglard J, Lagier G (1985) Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 40(2):111–118
Moore N, Kreft-Jais C, Haramburu F, Noblet C, Andrejak M, Ollagnier M et al (1997) Reports of hypoglycaemia associated with the use of ACE inhibitors and other drugs: a case/non-case study in the French pharmacovigilance system database. Br J Clin Pharmacol 44(5):513–518
Tubert P, Bégaud B, Haramburu F, Péré JC (1991) Spontaneous reporting: how many cases are required to trigger a warning? Br J Clin Pharmacol 32(4):407–408
Tubert-Bitter P, Begaud B, Moride Y, Chaslerie A, Haramburu F (1996) Comparing the toxicity of two drugs in the framework of spontaneous reporting: a confidence interval approach. J Clin Epidemiol 49(1):121–3
Fourrier A, Bégaud B, Alpérovitch A, Verdier-Taillefer MH, Touzé E, Decker N et al (2001) Hepatitis B vaccine and first episodes of central nervous system demyelinating disorders: a comparison between reported and expected number of cases. Br J Clin Pharmacol 51(5):489–490
Brown EG, Wood L, Wood S (1999) The medical dictionary for regulatory activities (MedDRA). Drug Saf 20(2):109–117
Miller GC, Britt H (1995) A new drug classification for computer systems: the ATC extension code. Int J Biomed Comput 40(2):121–124
European Medicines Agency (2009) EudraVigilance-Human Status Report [internet]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/10/WC500097692.pdf. Accessed 17 June 2011
Acknowledgements
We thank all the Regional Pharmacovigilance Centres who participated in this study
Funding
None.
Conflicts of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Pizzoglio, V., Ahmed, I., Auriche, P. et al. Implementation of an automated signal detection method in the French pharmacovigilance database: a feasibility study. Eur J Clin Pharmacol 68, 793–799 (2012). https://doi.org/10.1007/s00228-011-1178-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1178-1