Abstract
Background
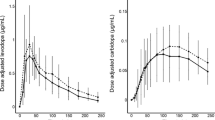
Repeated dosing of levodopa/carbidopa/entacapone (LCE) has shown a favourable pharmacokinetic (PK) profile compared with levodopa/carbidopa (LC), but increases maximum plasma levodopa concentrations (Cmax) during the day. High levodopa concentrations are associated with peak-dose dyskinesias.
Purpose
To determine whether administering LCE 75/18.5/200 and 125/31.5/200 mg (LCE 75 and LCE 125) following a morning dose of LCE 100/25/200 and 150/37.5/200 mg (LCE 100 and LCE 150), respectively, would avoid the increase in levodopa Cmax values observed during multiple dosing of LCE 100 and LCE 150.
Methods
This was an open, randomized, three-period, crossover PK trial in healthy volunteers (n = 19). Subjects were randomized to Group 1 or 2. Group 1 received in random sequence: LCE 150 followed by LCE 125 three times daily (tid); LCE 150 four times daily (qid); LC 150 qid. Group 2 received in random sequence: LCE 100 followed by LCE 75 tid; LCE 100 qid; LC 100 qid. Levodopa Cmax, minimum plasma concentration (Cmin), area under the curve (AUC0−14) and peak–trough fluctuation (PTF) were calculated up to 14 h after the first dose.
Results
Levodopa Cmax was not increased when the LCE dose was decreased by 25 mg after the morning dose. In comparison to LC, levodopa Cmin levels and AUC0−14 values for LCE were significantly higher, while the levodopa PTF was significantly smaller.
Conclusions
Reducing the dose of LCE by 25 mg following the first morning dose results in a more consistent levodopa Cmax profile, avoiding levodopa accumulation while maintaining significantly higher Cmin levels and AUC0−14 values compared with LC.


Similar content being viewed by others
References
Lewitt PA (2008) Levodopa for the treatment of Parkinson's disease. N Engl J Med 359(23):2468–2476
Hauser RA, McDermott MP, Messing S (2006) Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol 63(12):1756–1760
Fahn S (2000) The spectrum of levodopa-induced dyskinesias. Ann Neurol 47[4 Suppl 1]:S2–S9, discussion S9-S11
Tolosa ES, Martin WE, Cohen HP, Jacobson RL (1975) Patterns of clinical response and plasma dopa levels in Parkinson's disease. Neurology 25(2):177–183
Parkinson Study Group (1997) Entacapone improves motor fluctuations in levodopa-treated Parkinson's disease patients. Ann Neurol 42(5):747–755
Rinne UK, Larsen JP, Siden A, Worm-Petersen J (1998) Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. NOMECOMT Study Group. Neurology 51(5):1309–1314
Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M (2002) Efficacy and safety of entacapone in Parkinson's disease patients with suboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurol Scand 105(4):245–255
Brooks DJ, Sagar H (2003) Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson's disease: a randomised, placebo controlled, double blind, six month study. J Neurol Neurosurg Psychiatry 74(8):1071–1079
Keränen T, Gordin A, Harjola VP, Karlsson M, Korpela K, Pentikäinen PJ, Rita H, Seppalä L, Wikberg T (1993) The effect of catechol-O-methyltransferase inhibition by entacapone on the pharmacokinetics and metabolism of levodopa in healthy volunteers. Clin Neuropharmacol 16(2):145–156
Kuoppamäki M, Korpela K, Marttila R, Kaasinen V, Hartikainen P, Lyytinen J, Kaakkola S, Hänninen J, Löyttyniemi E, Kailajärvi M, Ruokoniemi P, Ellmén J (2009) Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa/carbidopa when administered four- or five-times daily. Eur J Clin Pharmacol 65(5):443–455
Müller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Woitalla D (2006) Inhibition of catechol-O-methyltransferase contributes to more stable levodopa plasma levels. Mov Disord 21(3):332–336
Müller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Goetze O, Woitalla D (2006) Pharmacokinetic behaviour of levodopa and 3-O-methyldopa after repeat administration of levodopa/carbidopa with and without entacapone in patients with Parkinson's disease. J Neural Transm 113:1441–1448
Ruottinen HM, Rinne UK (1996) A double-blind pharmacokinetic and clinical dose-response study of entacapone as an adjuvant to levodopa therapy in advanced Parkinson's disease. Clin Neuropharmacol 19(4):283–296
Müller T, Woitalla D, Saft C, Kuhn W (2000) Levodopa in plasma correlates with body weight of parkinsonian patients. Parkinsonism Relat Disord 6(3):171–173
Acknowledgements
The authors would like to thank Nadia Hashash, PhD, for editorial support in preparation of the manuscript. Editorial support was funded by Orion Corporation, Orion Pharma and Novartis Pharmaceuticals AG.
Conflicts of interest
All authors are employees of Orion Pharma.
Author information
Authors and Affiliations
Corresponding author
Additional information
EudraCT number: 2009-015625-37
Rights and permissions
About this article
Cite this article
Ingman, K., Naukkarinen, T., Vahteristo, M. et al. The effect of different dosing regimens of levodopa/carbidopa/entacapone on plasma levodopa concentrations. Eur J Clin Pharmacol 68, 281–289 (2012). https://doi.org/10.1007/s00228-011-1121-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1121-5