Abstract
Purpose
To evaluate the effects of two major polymorphisms of CYP2C9, CYP2C9*3 and CYP2C9*13, on the pharmacokinetics of irbesartan in healthy Korean volunteers.
Methods
A single 150-mg oral dose of irbesartan was given to 28 Korean volunteers, who had different CYP2C9 genotypes (12, 10, and 6 carriers of CYP2C9*1/*1, *1/*3, and *1/*13 genotypes respectively). Irbesartan levels were analyzed using HPLC fluorescence in plasma samples collected up to 36 h after the drug intake.
Results
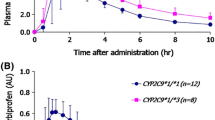
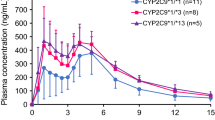
Compared with CYP2C9*1 homozygous subjects, not only were the maximum plasma concentrations (Cmax) of irbesartan in CYP2C9*1/*3 and *1/*13 subjects 1.56- and 1.50-fold higher (P = 0.001), but the half-lives were also 1.38- and 1.50-fold longer (P = 0.001). The area under the plasma concentration–time curve (AUC) was 1.64- and 1.79-fold higher (P < 0.001). The oral clearance of irbesartan was 39.3% and 44.0% lower in the CYP2C9*1/*3 and *1/*13 subjects respectively, than in the *1/*1 subjects (P < 0.001). Likewise, the increases in half-life and decreases in oral clearance observed in CYP2C9*1/*13 individuals were similar to those in participants expressing the CYP2C9*1/*3 genotype.
Conclusions
CYP2C9 genetic polymorphisms markedly affected the pharmacokinetics of irbesartan in this study sample. The CYP2C9*3 and CYP2C9*13 alleles appear to be associated with the decreased metabolism of irbesartan.

Similar content being viewed by others
References
Marino MR, Vachharajani NN (2001) Drug interactions with irbesartan. Clin Pharmacokinet 40:605–614
Perrier L, Bourrié M, Marti E, Tronquet C, Massé D, Berger Y, Magdalou J, Fabre G (1994) In vitro N-glucuronidation of SB 47436 (BMS 186295), a new AT1 nonpeptide angiotensin II receptor antagonist, by rat, monkey and human hepatic microsomal fractions. J Pharmacol Exp Ther 271:91–99
Bourrié M, Meunier V, Berger Y, Fabre G (1999) Role of cytochrome P-4502C9 in irbesartan oxidation by human liver microsomes. Drug Metab Dispos 27:288–296
Gillis JC, Markham A (1997) Irbesartan. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in the management of hypertension. Drugs 54:885–902
Marino MR, Langenbacher K, Ford NF, Uderman HD (1998) Pharmacokinetics and pharmacodynamics of irbesartan in healthy subjects. J Clin Pharmacol 38:246–255
Van Booven D, Marsh S, McLeod H, Carrillo MW, Sangkuhl K, Klein TE, Altman RB (2010) Cytochrome P450 2C9-CYP2C9. Pharmacogenet Genomics 20:277–281
Miners JO, Birkett DJ (1998) Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45:525–538
Lee CR, Goldstein JA, Pieper JA (2002) Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 12:251–263, Erratum in: (2002) Pharmacogenetics 12:343
Guo Y, Zhang Y, Wang Y, Chen X, Si D, Zhong D, Fawcett JP, Zhou H (2005) Role of CYP2C9 and its variants (CYP2C9*3 and CYP2C9*13) in the metabolism of lornoxicam in humans. Drug Metab Dispos 33:749–753
Li Z, Wang G, Wang LS, Zhang W, Tan ZR, Fan L, Chen BL, Li Q, Liu J, Tu JH, Hu DL, Liu ZQ, Zhou HH (2009) Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica 39:788–793
Bae JW, Choi CI, Jang CG, Lee SY (2011) Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam. Br J Clin Pharmacol 71:550–555
García-Martín E, Martínez C, Ladero JM, Agúndez JA (2006) Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther 10:29–40
Nakai K, Habano W, Nakai K, Fukushima N, Suwabe A, Moriya S, Osano K, Gurwitz D (2005) Ethnic differences in CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu) genotypes in Japanese and Israeli populations. Life Sci 78:107–111
Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, Lee SY (2005) Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol 60:418–422
Si D, Guo Y, Zhang Y, Yang L, Zhou H, Zhong D (2004) Identification of a novel variant CYP2C9 allele in Chinese. Pharmacogenetics 14:465–469
Maekawa K, Harakawa N, Sugiyama E, Tohkin M, Kim SR, Kaniwa N, Katori N, Hasegawa R, Yasuda K, Kamide K, Miyata T, Saito Y, Sawada J (2009) Substrate-dependent functional alterations of seven CYP2C9 variants found in Japanese subjects. Drug Metab Dispos 37:1895–1903
Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ (2010) Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 11:781–791
Dupont WD, Plummer WD (1998) Power and sample size calculations for studies involving linear regression. Control Clin Trials 19:589–601
Kirchheiner J, Bauer S, Meineke I, Rohde W, Prang V, Meisel C, Roots I, Brockmöller J (2002) Impact of CYP2C9 and CYP2C19 polymorphisms on tolbutamide kinetics and the insulin and glucose response in healthy volunteers. Pharmacogenetics 12:101–109
Scordo MG, Pengo V, Spina E, Dahl ML, Gusella M, Padrini R (2002) Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther 72:702–710
Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA, Goldstein JA (2003) Tolbutamide, flurbiprofen, and losartan as probes of CYP2C9 activity in humans. J Clin Pharmacol 43:84–91
Zhou YH, Zheng QC, Li ZS, Zhang Y, Sun M, Sun CC, Si D, Cai L, Guo Y, Zhou H (2006) On the human CYP2C9*13 variant activity reduction: a molecular dynamics simulation and docking study. Biochimie 88:1457–1465
Guo Y, Wang Y, Si D, Fawcett PJ, Zhong D, Zhou H (2005) Catalytic activities of human cytochrome P450 2C9*1, 2C9*3 and 2C9*13. Xenobiotica 35:853–861
Hong X, Zhang S, Mao G, Jiang S, Zhang Y, Yu Y, Tang G, Xing H, Xu X (2005) CYP2C9*3 allelic variant is associated with metabolism of irbesartan in Chinese population. Eur J Clin Pharmacol 61:627–634
Chen G, Jiang S, Mao G, Zhang S, Hong X, Tang G, Li Z, Liu X, Zhang Y, Xing H, Wang B, Yu Y, Xu X (2006) CYP2C9 Ile359Leu polymorphism, plasma irbesartan concentration and acute blood pressure reductions in response to irbesartan treatment in Chinese hypertensive patients. Methods Find Exp Clin Pharmacol 28:19–24
Hallberg P, Karlsson J, Kurland L, Lind L, Kahan T, Malmqvist K, Ohman KP, Nyström F, Melhus H (2002) The CYP2C9 genotype predicts the blood pressure response to irbesartan: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation vs Atenolol (SILVHIA) trial. J Hypertens 20:2089–2093
Wen SY, Wang H, Sun OJ, Wang SQ (2003) Rapid detection of the known SNPs of CYP2C9 using oligonucleotide microarray. World J Gastroenterol 9:1342–1346
Acknowledgements
This study was supported by a grant from the Korean Food and Drug Administration. We thank Ho-Kyun Han, Kyeong-Joo Jeon, So-Jung Youn, Seul-Ki Keum, and Da-Hee Oh for their help with clinical study and subject genotyping.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chang-Ik Choi and Mi-Jeong Kim contributed equally to this article.
Rights and permissions
About this article
Cite this article
Choi, CI., Kim, MJ., Chung, EK. et al. CYP2C9*3 and *13 alleles significantly affect the pharmacokinetics of irbesartan in healthy Korean subjects. Eur J Clin Pharmacol 68, 149–154 (2012). https://doi.org/10.1007/s00228-011-1098-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1098-0