Abstract
Purpose
After reports of malaise in infants immediately after the oral administration of two brands of vitamin D solutions, a “Dear Doctor letter” (DDL) containing recommendations for the administration of vitamin D was sent to all French paediatricians and pharmacies and a large number of French general practitioners (GPs) with a predominantly paediatric practice. The DDL and a press release were published on the French Medicines Agency website and distributed via a mailing list. The objective of this study was to assess the effectiveness of such a DDL and to collect the opinions of healthcare professionals on the best way to provide them with information.
Methods
A questionnaire was sent to a national random sample of 145 paediatricians, 680 GPs and 230 pharmacists.
Results
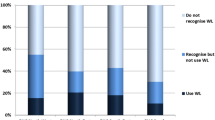
Only 49% of responding paediatricians, 48% of GPs and 67% of pharmacists were aware of the warning. Among the participating healthcare professionals aware of the warning and who prescribed/dispensed these vitamins, 50% of paediatricians and 68% of GPs stated that they had changed their prescribing behaviour, and 68% of pharmacists stated that they had modified their advice when dispensing. According to the responding healthcare professionals, postal letters remained the best way to issue safety warnings. Some of the respondents suggested that the DDL be more distinctive in terms of being a DDL and that the information be more widely disseminated to other stakeholders involved in the healthcare system.
Conclusions
This survey of a national random sample of healthcare professionals revealed that many of the respondents paid little attention to the DDL and were therefore unlikely to change their practices. A potential supplementary method for disseminating recommendations for medicine administration could be to apply stickers on medicine boxes, as this approach has the additional advantage of directly informing the concerned population, i.e. the parents.

Similar content being viewed by others
References
EMEA Guideline on risk management systems for medicinal products for human use (CHMP) (2005) Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/2010/WC500004888.pdf. Accessed Feb 2011
Volume 9A of The Rules Governing Medicinal Products in the European Union (2006) Guidelines on Pharmacovigilance for Medicinal Products for Human Use (2008). Available at: http://ec.europa.eu/health/files/pharmacos/docs/doc2005/2012-2005/draft_of_volume_2009a_2012_2005_en.pdf. Accessed Feb 2011
Lettre aux prescripteurs adressée par les laboratoires Crinex (2006) Information importante de pharmacovigilance Uvestérol Vitamine ADEC, solution buvable Uvestérol D 1500UI/mL, solution buvable. Available at: http://www.afssaps.fr/content/download/11927/142830/version/142832/file/ddl_uvesterol.pdf. Accessed Feb 2011
Communiqué de presse de l'AFSSAPS (2006) Nouvelles recommandations d'administration pour Uvestérol vitaminé ADEC et Uvestérol D 1500UI/mL. http://www.afssaps.fr/Infos-de-securite/Communiques-de-presse/Nouvelles-recommandations-d-administration-pour-Uvesterol-Vitamine-ADEC-et-Uvesterol-D-1500UI-mL. Accessed Feb 2011
Mol PG, Straus SM, Piening S, de Vries JT, de Graeff PA, Haaijer-Ruskamp FM (2010) A decade of safety-related regulatory action in the Netherlands: a retrospective analysis of direct healthcare professional communications from 1999 to 2009. Drug Saf 33:463–474 4. doi:10.2165/11532840-000000000-00000
MHRA Safety warnings and messages for medicines. Available at:http://www.mhra.gov.uk/Safetyinformation. Accessed Feb 2011
AFSSAPS (Lettre aux professionels de santé). Available at: http://www.afssaps.fr/Infos-de-securite/Lettres-aux-professionnels-de-sante. Accessed Feb 2011
van Grootheest AC, Edwards IR (2002) Labelling and 'Dear Doctor' letters: are they noncommittal? Drug Saf 25:1051–1055
Sondergaard J, Andersen M, Vach K, Kragstrup J, Maclure M, Gram LF (2002) Detailed postal feedback about prescribing to asthma patients combined with a guideline statement showed no impact: a randomised controlled trial. Eur J Clin Pharmacol 58:127–132. doi:10.1007/s00228-002-0454-5
Smalley W, Shatin D, Wysowski DK, Gurwitz J, Andrade SE, Goodman M, Chan KA et al (2000) Contraindicated use of cisapride: impact of food and drug administration regulatory action. JAMA 284:3036–3039
Shatin D, Gardner JS, Stergachis A, Blough D, Graham D (2005) Impact of mailed warning to prescribers on the co-prescription of tramadol and antidepressants. Pharmacoepidemiol Drug Saf 14:149–154
Hurault C, Lacroix I, Bourrel R, Montastruc JL, Damase-Michel C (2008) Writing and dispensing of NSAID prescriptions in late pregnancy: impact of health authorities' advice. Presse Méd 37:767–774
Graham DJ, Drinkard CR, Shatin D, Tsong Y, Burgess MJ (2001) Liver enzyme monitoring in patients treated with troglitazone. JAMA 286:831–833
Cluxton RJ Jr, Li Z, Heaton PC, Weiss SR, Zuckerman IH, Moomaw CJ, Hsu VD et al (2005) Impact of regulatory labeling for troglitazone and rosiglitazone on hepatic enzyme monitoring compliance: findings from the state of Ohio medicaid program. Pharmacoepidemiol Drug Saf 14:1–9
Bahri P (2010) Public pharmacovigilance communication: a process calling for evidence-based, objective-driven strategies. Drug Saf 33:1065–1079. doi:10.2165/11539040-000000000-00000
Guo JJ, Curkendall S, Jones JK, Fife D, Goehring E, She D (2003) Impact of cisapride label changes on codispensing of contraindicated medications. Pharmacoepidemiol Drug Saf 12:295–301
Victorri-Vigneau C, Marais M, Veyrac G, Chaslerie A, Pivette J, Jolliet P (2007) Reactivity and communication of Health Authorities toward health professionals: a public health priority. Therapie 62:513–517
Weatherby LB, Walker AM, Fife D, Vervaet P, Klausner MA (2001) Contraindicated medications dispensed with cisapride: temporal trends in relation to the sending of 'Dear Doctor' letters. Pharmacoepidemiol Drug Saf 10:211–218
Sturkenboom MC, de Jong-van den Berg LT, Cornel MC, Stricker BH, Wesseling H (1994) Communicating a drug alert. A case study on acitretin in The Netherlands. Eur J Clin Pharmacol 47:125–132
Volume 9A of The Rules Governing Medicinal Products in the European Union (2008) Guidelines on Pharmacovigilance for Medicinal Products for Human Use. Available at; http://ec.europa.eu/health/files/eudralex/vol-9/pdf/vol9a_09-2008_en.pdf. Accessed Feb 2011
Sanfelix-Gimeno G, Cervera-Casino P, Peiro S, Lopez-Valcarcel BG, Blazquez A, Barbera T (2009) Effectiveness of safety warnings in atypical antipsychotic drugs: an interrupted time-series analysis in Spain. Drug Saf 32:1075–108. doi:10.2165/11316520-000000000-00000
Carlson AM, Morris LS (1996) Coprescription of terfenadine and erythromycin or ketoconazole: an assessment of potential harm. J Am Pharm Assoc Wash NS36:263–269
Jones JK, Fife D, Curkendall S, Goehring E Jr, Guo JJ, Shannon M (2001) Coprescribing and codispensing of cisapride and contraindicated drugs. JAMA 286:1607–1609
Lee LY, Kortepeter CM, Willy ME, Nourjah P (2008) Drug-risk communication to pharmacists: assessing the impact of risk-minimization strategies on the practice of pharmacy. J Am Pharm Assoc 48:494–500
Wilkinson JJ, Force RW, Cady PS (2004) Impact of safety warnings on drug utilization: marketplace life span of cisapride and troglitazone. Pharmacotherapy 24:978–986
EMEA/CPMP (2005)Working Group with Patients Organisations. Outcome of discussions: recommendations and proposals for action. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/partners_and_networks/document_listing/document_listing_000235.jsp&murl=menus/partners_and_networks/partners_and_networks.jsp&mid=WC0b01ac05800aa3cb&jsenabled=true#. Accessed Feb 2011
Orme M (2002) The Erice Statement on drug advertising to consumers. Eur J Clin Pharmacol 58:S106–108
Hugman B, Edwards IR (2006) The challenge of effectively communicating patient safety information. Expert Opin Drug Saf 5:495–499. doi:10.1517/14740338.5.4.495
Commission nationale de l'informatique et des libertés. Available at: http://www.cnil.fr. Accessed February 2011.
Power A, Johnson BJ, Diack HL, McKellar S, Stewart D, Hudson SA (2008) Scottish pharmacists' views and attitudes towards continuing professional development. Pharm World Sci 30:136–143. doi:10.1007/s11096-007-9156-5
Kaestner SA, Sewell GJ (2009) A national survey investigating UK prescribers' opinions on chemotherapy dosing and 'dose-banding'. Clin Oncol R Coll Radiol 21:320–328. doi:10.1016/j.clon.2008.12.004
Acknowledgements
We thank the physician and pharmacist professional bodies that performed the random selection, as well as all those who participated to this study.
Funding
None
Competing interests
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Théophile, H., Miremont-Salamé, G., Robinson, P. et al. Relevance of a “Dear Doctor letter” to alert healthcare providers to new recommendations for vitamin D administration. Eur J Clin Pharmacol 67, 681–686 (2011). https://doi.org/10.1007/s00228-011-1055-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1055-y