Abstract
Purpose
Letrozole is an orally active aromatase inhibitor for the treatment of breast cancer. The objectives of this study were to examine the pharmacokinetic profile of letrozole in Japanese subjects and to identify factors that influence variability in the pharmacokinetics of letrozole using population pharmacokinetic (PPK) analysis.
Methods
Twenty-five healthy postmenopausal Japanese women were enrolled in the study and received 2.5 mg letrozole once daily for 14 or 28 days. A PPK model was developed using NONMEM software. Age, body weight (WT), AST, ALT, total bilirubin, serum creatinine (CRE), and genotype of CYP2A6 were studied as covariates. Estrone, estrone sulfate, and estradiol in plasma were measured as pharmacodynamic markers.
Results
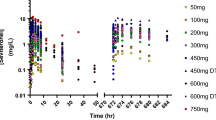
CYP2A6 genotype, CRE, and AST were significant covariates for apparent systemic clearance (CL/F), and WT was a significant covariate for apparent distribution volume (Vd/F). Population mean estimates of CL/F and Vd/F in subjects without CYP2A6 mutation were 1.03 × (CRE/0.70)−1.27 × (AST/17.5)−0.793 L/h and 94.2 × (WT/51.1)1.12 L respectively. CL/F in subjects possessing 1 and 2 CYP2A6 mutation alleles were 84.3% and 44.8% of the value in the subjects without mutation respectively. Estrogen levels fell to below detection limits in most subjects after letrozole administration. Three mild and transient adverse events (upper respiratory tract inflammation, arthralgia, and vomiting) were reported in the study.
Conclusions
CYP2A6 genotype largely influences CL/F of letrozole. Genetic polymorphism of CYP2A6 and body weight will be causes of ethnic difference in PK. However, dose adjustment is not necessary, because of the wide therapeutic range.

Similar content being viewed by others
References
Yager JD, Davidson NE (2006) Estrogen carcinogenesis in breast cancer. N Engl J Med 354:270–282
Johnston SR, Dowsett M (2003) Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer 3:821–831
Osborne CK (1998) Tamoxifen in the treatment of breast cancer. N Engl J Med 339:1609–1618
Guengerich FP (2003) Cytochromes P450, drugs, and diseases. Mol Interv 3:194–204
Amar S, Roy V, Perez EA (2007) Letrozole: present and future role in the treatment of breast cancer. Expert Opin Pharmacother 8:1965–1975
Brodie A (2002) Aromatase inhibitors in breast cancer. Trends Endocrinol Metab 13:61–65
Sioufi A, Gauducheau N, Pineau V, Marfil F, Jaouen A, Cardot JM, Godbillon J, Czendlik C, Howald H, Pfister C, Vreeland F (1997) Absolute bioavailability of letrozole in healthy postmenopausal women. Biopharm Drug Dispos 18:779–789
Pfister CU, Martoni A, Zamagni C, Lelli G, De Braud F, Souppart C, Duval M, Hornberger U (2001) Effect of age and single versus multiple dose pharmacokinetics of letrozole (Femara) in breast cancer patients. Biopharm Drug Dispos 22:191–197
Murai K, Yamazaki H, Nakagawa K, Kawai R, Kamataki T (2009) Deactivation of anti-cancer drug letrozole to a carbinol metabolite by polymorphic cytochrome P450 2A6 in human liver microsomes. Xenobiotica 39:795–802
Nakajima M, Kuroiwa Y, Yokoi T (2002) Interindividual differences in nicotine metabolism and genetic polymorphisms of human CYP2A6. Drug Metab Rev 34:865–877
Daigo S, Takahashi Y, Fujieda M, Ariyoshi N, Yamazaki H, Koizumi W, Tanabe S, Saigenji K, Nagayama S, Ikeda K, Nishioka Y, Kamataki T (2002) A novel mutant allele of the CYP2A6 gene (CYP2A6*11) found in a cancer patient who showed poor metabolic phenotype towards tegafur. Pharmacogenetics 12:299–306
Xu C, Rao YS, Xu B, Hoffmann E, Jones J, Sellers EM, Tyndale RF (2002) An in vivo pilot study characterizing the new CYP2A6*7, *8, and *10 alleles. Biochem Biophys Res Commun 290:318–324
Marfil F, Pineau V, Sioufi A, Godbillon SJ (1996) High-performance liquid chromatography of the aromatase inhibitor, letrozole, and its metabolite in biological fluids with automated liquid-solid extraction and fluorescence detection. J Chromatogr B Biomed Appl 683:251–258
Lipton A, Demers LM, Harvey HA, Kambic KB, Grossberg H, Brady C, Adlercruetz H, Trunet PF, Santen RJ (1995) Letrozole (CGS 20267). A phase I study of a new potent oral aromatase inhibitor of breast cancer. Cancer 15:2132–2138
Nevilie M, Selzer R, Aizenstein B, Maguire M, Hogan K, Walton R, Welsh K, Neri B, de Arruda M (2002) Characterization of cytochrome P450 2D6 alleles using the Invader system. Biotechniques 32 [Suppl]:34–43
Wirz B, Valles B, Parkinson A, Madan A, Probst A, Zimmerlin A, Gut J (1996) CYP3A4 and CYP2A6 are involved in the biotransformation of letrozole. Proceedings of 7th North American ISSX Meeting 10:359
Pichette V, Leblond FA (2003) Drug metabolism in chronic renal failure. Curr Drug Metab 4:91–103
Sun H, Frassetto L, Benet LZ (2006) Effects of renal failure on drug transport and metabolism. Pharmacol Ther 109:1–11
Pfister CU, Horak J, Horejsova M, Cieslarova B, Hasa J, Heine P, On N, Souppart C, Schaeffer N (2001) Pharmacokinetics of Femara in subjects with severe liver impairment. Proceedings of American Association for Cancer Research 42:544
Nabholtz JM (2008) Aromatase inhibitors in the management of early breast cancer. Eur J Surg Oncol 34:1199–1207
Chung CT, Carlson RW (2003) The role of aromatase inhibitors in early breast cancer. Curr Treat Options Oncol 4:133–140
Wolff AC (2002) Systemic therapy. Curr Opin Oncol 14:600–608
Dixon JM, Love CD, Bellamy CO, Cameron DA, Leonard RC, Smith H, Miller WR (2001) Letrozole as primary medical therapy for locally advanced and large operable breast cancer. Breast Cancer Res Treat 66:191–199
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanii, H., Shitara, Y. & Horie, T. Population pharmacokinetic analysis of letrozole in Japanese postmenopausal women. Eur J Clin Pharmacol 67, 1017–1025 (2011). https://doi.org/10.1007/s00228-011-1042-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1042-3