Abstract
Objective
This study compares midazolam with omeprazole as marker drugs for the evaluation of CYP3A activity in nine healthy self-reported white Brazilian volunteers.
Methods
Omeprazole was also used to evaluate the CYP2C19 phenotype. The volunteers received p.o. 20 mg omeprazole, and blood samples were collected 3.5 h after drug administration. After a washout period of 10 days, the volunteers received p.o. 15 mg midazolam maleate, and serial blood samples were collected up to 6 h after administration of the drug. CYP2C19 was genotyped for the allelic variants CYP2C19*1, CYP2C19*2, CYP2C19*3, and CYP2C19*17. Analysis of omeprazole, hydroxyomeprazole, omeprazole sulfone, and midazolam in plasma was carried out by LC-MS/MS.
Results
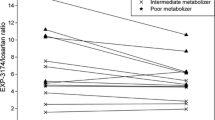
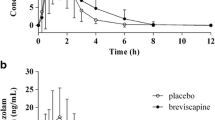
The volunteers genotyped as CYP2C19*1*17, CYP2C19*17*17, CYP2C19*1*1 (n = 8), or CYP2C19*17*2 (n = 1) presented a median hydroxylation index (omeprazole/hydroxyomeprazole) of 1.35, indicating that all of them were extensive metabolizers of CYP2C19. The volunteers (n = 9) presented a 0.12 log of the omeprazole/sulfone ratio and a median oral clearance of midazolam of 17.89 ml min−1 kg−1, suggesting normal CYP3A activity.
Conclusions
Orthogonal regression analysis between midazolam clearance and log of the plasma concentrations of the omeprazole/omeprazole sulfone ratio (R = −0.7544, P < 0.05) suggests that both midazolam and omeprazole can be used as markers of CYP3A activity in the population investigated.

Similar content being viewed by others
References
Ingelman-Sundberg M, Rodriguez-Antona C (2005) Pharmacogenetics of drug-metabolizing enzymes: implications for a safer and more effective drug therapy. Phil Trans R Soc B 360:1563–1570
Brosen K, Naranjo CA (2001) Review of pharmacokinetic and pharmacodynamic interaction studies with citalopram. Eur Neuropsychopharmacol 11:275–283
Ingelman-Sundberg M, Orcarson M, Mclellan RA (1999) Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Tips 20:342–349
Hasler JA, Estabrook R, Murray M, Pikuleva I, Waterman M, Capdevila J, Holla V, Helvig C, Falck JR, Farrel G, Kaminsky LS, Spirovack SD, Boitier E, Beaune P (1999) Human cytochromes P450. Mol Aspects Med 20:1–137
Rossini A, Soares-Lima S, Rapozo DCM, Faria M, Albano RM, Ribeiro Pinto LF (2006) CYP2A6 and CYP2E1 polymorphisms in a Brazilian population living in Rio de Janeiro. Braz J Med Biol Res 39:195–201
Suarez-Kurtz G (2005) Pharmacogenomics in admixed populations. Trends Pharmacol Sci 26:196–201
Carvalho-Silva D, Santos FR, Rocha J, Pena SDJ (2001) The phylogeography of Brazilian Y-chromosome lineages. Am J Hum Genet 68:281–286
Daly AK (2006) Significance of the minor cytochrome P450 3A isoforms. Clin Pharmacokinet 45:13–31
Rogers JF, Rocci ML, Haughey DB, Bertino JS (2003) An evaluation of the suitability of intravenous midazolam as an in vivo marker for hepatic cytocrome P4503A activity. Clin Pharmacol Therap 73:153–158
Tateishi T, Watanabe M, Nakura H, Asoh M, Shirai H, Mizorogi Y, Kobayashi S, Thummel KE, Wilkinson GR (2001) CYP3A activity in European American and Japanese men using midazolam as an in vivo probe. Clin Pharmacol Ther 69:333–339
Kim M-J, Nafziger AN, Zhang Y, Sellers EM, Gaedigk A, Bertino JS Jr (2004) Lack of weight-based dose dependency and intraindividual variability of omeprazole for CYP2C19 phenotyping. J Clin Pharmacol 44:966–973
Rosemary J, Adithan C, Padmaja N, Shashindran CH, Gerard N, Krishnamoorthy R (2005) The effect of the CYP2C19 genotype on the hydroxylation index of omeprazole in south Indians. Eur J Clin Pharmacol 61:19–23
Marinac JS, Balian J, Foxworth JW, Willsie SK, Daus JC, Owen R, Flockhart DA (1996) Determination of CYP2C19 phenotype in black Americans with omeprazole: correlation with genotype. Clin Pharmacol Ther 60:138–144
Rudberg I, Mohebi B, Hermann M, Refsum H, Molden E (2008) Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther 83:322–327
Sim SC, Risinger C, Dahl M-L, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
González HM, Romero EM, Chavez TJ, Peregrina AA, Chávez TJ, Escobar-Islas E, Lozano F, Hoyo-Vadillo C (2003) CYP2C19 and CYP3A4-dependent omeprazole metabolism in west Mexicans. J Clin Pharmacol 43:1211–1215
González HM, Romero EM, Chavez TJ, Peregrina AA, Quezada V, Hoyo-Vadillo C (2002) Phenotype of CYP2C19 and CYP3A4 by determination of omeprazole and its two main metabolites in plasma using liquid chromatography with liquid-liquid extraction. J Chromatogr B 780:459–465
Kanazawa H, Okada A, Higaki M, Yokota H, Mashige F, Nakahara K (2003) Stereospecific analysis of omeprazole in human plasma as a probe for CYP2C19 phenotype. J Pharm Biomed Anal 30:1817–1824
Yin OQP, Tomlinson B, Chow AHL, Waye MM, Chow MSS (2004) Omeprazole as a CYP2C19 marker in Chinese subjects: assessment of its gene-dose effect and intrasubject variability. J Clin Pharmacol 44:582–589
Bottiger Y (2006) Use of omeprazole sulfone in a single plasma sample as a probe for CYP3A4. Eur J Clin Pharmacol 62:621–625
Goldstein JA, Blaisdell J (1996) Genetic tests which identify the principal defects in CYP2C19 responsible for the polymorphism in mephenytoin metabolism. Methods Enzymol 272:210–218
Jabor VAP, Coelho EB, Santos NAG, Bonato PS, Lanchote VL (2005) A highly sensitive LC-MS-MS assay for analysis of midazolam and its major metabolite in human plasma: applications to drug metabolism. J Chromatogr B 822:27–32
Schellens JHM, Van Der Wart JHF, Danhof M, Van Der Velde EA (1988) Relationship between the metabolism of antipyrine, hexobarbitone and theophyline in man as assessed by a cocktail approach. Br J Clin Pharmacol 26:373–384
Chang M, Tybring G, Dahl M-L, Gotharson E, Sagar M, Seensalu R, Bertilsson L (1995) Interphenotype differences in disposition and effect on gastrin levels of omeprazole-suitability of omeprazole as a probe for CYP2C19. Br J Clin Pharmacol 39:511–518
Yu B-N, Chen G-L, He N, Ouyang D-S, Chen X-P, Liu Z-Q, Zhou H-H (2003) Pharmacokinetics of citalopram in relation to genetic polymorphism of CYP2C19. Drug Metabol Dispos 31:1255–1259
Yang YS, Wong LP, Lee TC, Mustafa AM, Mohamed Z, Lang CC (2004) Genetic polymorphism of cytochrome P450 2C19 in healthy Malaysian subjects. Br J Clin Pharmacol 58:332–335
Lamba JK, Lin YS, Schuetz ER, Thummel KE (2002) Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv 54:1271–1294
Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Fundação de Apoio a Pesquisa e Assistência do HCFMRP-USP (FAEPA) and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support and for granting research fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocha, A., Coelho, E.B., Moussa, S.A.P. et al. Investigation of the in vivo activity of CYP3A in Brazilian volunteers: comparison of midazolam and omeprazole as drug markers. Eur J Clin Pharmacol 64, 901–906 (2008). https://doi.org/10.1007/s00228-008-0510-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0510-x