Abstract
Background and objective
We recently discovered that rofecoxib is a potent mechanism-based inhibitor of CYP1A2. The effect of the widely used cyclo-oxygenase-2 selective non-steroidal anti-inflammatory drug celecoxib on CYP1A2 activity has not been reported.
Methods
The effect of celecoxib on CYP1A2 activity (phenacetin O-deethylation) was first studied in vitro using human liver microsomes. This was followed by a randomized, placebo-controlled, cross-over study in which 12 healthy volunteers were given celecoxib (200 mg twice daily) or placebo for 4 days. On day 3, a caffeine test was performed. On day 4, the subjects ingested 2 mg tizanidine. Plasma samples for the measurement of the concentrations of tizanidine, its metabolites and celecoxib were collected up to 24 h post-administration. Pharmacodynamic variables (e.g. blood pressure, subjective drowsiness and drug effect) were recorded up to 12 h post-adm.
Results
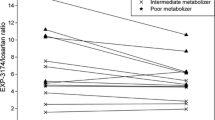
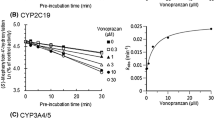
Celecoxib was found to be a moderately potent competitive inhibitor of CYP1A2 in vitro with a Ki (inhibitor constant) of 25.4 μM. However, in vivo, celecoxib did not affect the caffeine test, or the peak concentration, time to peak concentration, area under the concentration-time curve or half-life of tizanidine. The pharmacodynamic variables of tizanidine also remained unchanged.
Conclusions
Unlike rofecoxib, celecoxib does not clinically to significantly inhibit CYP1A2. The lack of significant in vivo inhibition of CYP1A2 can be correctly predicted on the basis of in vitro Ki data and the free peripheral or portal plasma concentration of celecoxib.




Similar content being viewed by others
References
Bachmann K, White D, Jauregui L, Schwartz JI, Agrawal NG, Mazenko R, Larson PJ, Porras AG (2003) An evaluation of the dose-dependent inhibition of CYP1A2 by rofecoxib using theophylline as a CYP1A2 probe. J Clin Pharmacol 43:1082–1090
Backman JT, Granfors MT, Neuvonen PJ (2006) Rifampicin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: studies with tizanidine and caffeine. Eur J Clin Pharmacol 62:451–461
Backman JT, Karjalainen MJ, Neuvonen M, Laitila J, Neuvonen PJ (2006) Rofecoxib is a potent inhibitor of cytochrome P450 1A2: studies with tizanidine and caffeine in healthy subjects. Br J Clin Pharmacol 62:345–357
Backman JT, Schröder MT, Neuvonen PJ (2008) Effects of gender and moderate smoking on the pharmacokinetics and effects of the CYP1A2 substrate tizanidine. Eur J Clin Pharmacol 64:17–24
Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA (2003) The conduct of in vitro and in vivo drug-drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos 31:815–832
Bradley CP (2007) Review: risk for cardiovascular events is increased with rofecoxib, diclofenac, and indomethacin but not celecoxib (comment). ACP J Club 146:24
Carrillo JA, Christensen M, Ramos SI, Alm C, Dahl ML, Benitez J, Bertilsson L (2000) Evaluation of caffeine as an in vivo probe for CYP1A2 using measurements in plasma, saliva, and urine. Ther Drug Monit 22:409–417
Chavez-Eng CM, Constanzer ML, Matuszewski BK (2000) Determination of rofecoxib (MK-0966), a cyclooxygenase-2 inhibitor, in human plasma by high-performance liquid chromatography with tandem mass spectrometric detection. J Chromatogr B Biomed Sci Appl 748:31–39
Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H (2006) Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 295:1135–1141
Davies NM, McLachlan AJ, Day RO, Williams KM (2000) Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet 38:225–242
Distlerath LM, Reilly PE, Martin MV, Davis GG, Wilkinson GR, Guengerich FP (1985) Purification and characterization of the human liver cytochromes P-450 involved in debrisoquine 4-hydroxylation and phenacetin O-deethylation, two prototypes for genetic polymorphism in oxidative drug metabolism. J Biol Chem 260:9057–9067
EMEA (2006) Celecoxib BILAG I PRODUKTRESUME. URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/onsenal/H-466-PI-da.pdf. Accessed 2 Oct 2007
Faber MS, Jetter A, Fuhr U (2005) Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol 97:125–134
FDA (1999) Celebrex (Celecoxib) Capsules Application No.: 21–156 & 20998/S007 Clinical Pharmacology and Biopharmaceutics reviews section. URL: http://www.fda.gov/CDER/foi/nda/99/21156-S007_Celebrex.htm. Accessed 27 Aug 2007
Fuhr U, Rost KL, Engelhardt R, Sachs M, Liermann D, Belloc C, Beaune P, Janezic S, Grant D, Meyer UA, Staib AH (1996) Evaluation of caffeine as a test drug for CYP1A2, NAT2 and CYP2E1 phenotyping in man by in vivo versus in vitro correlations. Pharmacogenetics 6:159–176
Granfors MT, Backman JT, Laitila J, Neuvonen PJ (2004) Tizanidine is mainly metabolized by cytochrome p450 1A2 in vitro. Br J Clin Pharmacol 57:349–353
Granfors MT, Backman JT, Laitila J, Neuvonen PJ (2005) Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2. Clin Pharmacol Ther 78:400–411
Granfors MT, Backman JT, Neuvonen M, Ahonen J, Neuvonen PJ (2004) Fluvoxamine drastically increases concentrations and effects of tizanidine: a potentially hazardous interaction. Clin Pharmacol Ther 75:331–341
Granfors MT, Backman JT, Neuvonen M, Neuvonen PJ (2004) Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther 76:598–606
Holland DT, Godfredsen KA, Page T, Connor JD (1998) Simple high-performance liquid chromatography method for the simultaneous determination of serum caffeine and paraxanthine following rapid sample preparation. J Chromatogr B Biomed Sci Appl 707:105–110
Jeppesen U, Gram LF, Vistisen K, Loft S, Poulsen HE, Brøsen K (1996) Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol 51:73–78
Karjalainen MJ, Neuvonen PJ, Backman JT (2006) Rofecoxib is a potent, metabolism-dependent inhibitor of CYP1A2: implications for in vitro prediction of drug interactions. Drug Metab Dispos 34:2091–2096
Karjalainen MJ, Neuvonen PJ, Backman JT (2007) Tolfenamic acid is a potent CYP1A2 inhibitor in vitro but does not interact in vivo: correction for protein binding is needed for data interpretation. Eur J Clin Pharmacol 63:829–836
Obach RS, Walsky RL, Venkatakrishnan K, Gaman EA, Houston JB, Tremaine LM (2006) The utility of in vitro cytochrome P450 inhibition data in the prediction of drug-drug interactions. J Pharmacol Exp Ther 316:336–348
Obach RS, Walsky RL, Venkatakrishnan K, Houston JB, Tremaine LM (2005) In vitro cytochrome P450 inhibition data and the prediction of drug-drug interactions: qualitative relationships, quantitative predictions, and the rank-order approach. Clin Pharmacol Ther 78:582–592
Oitate M, Hirota T, Murai T, Miura S, Ikeda T (2007) Covalent binding of rofecoxib, but not other cyclooxygenase-2 inhibitors, to allysine aldehyde in elastin of human aorta. Drug Metab Dispos 35:1846–1852
Paulson SK, Kaprak TA, Gresk CJ, Fast DM, Baratta MT, Burton EG, Breau AP, Karim A (1999) Plasma protein binding of celecoxib in mice, rat, rabbit, dog and human. Biopharm Drug Dispos 20:293–299
Pelkonen O, Mäenpää J, Taavitsainen P, Rautio A, Raunio H (1998) Inhibition and induction of human cytochrome P450 (CYP) enzymes. Xenobiotica 28:1203–1253
Pfizer, Searle (2005) CELEBREX celecoxib capsules (Product information). URL: http://www.fda.gov/cder/foi/label/2005/020998s018,019lbl.pdf. Accessed 22 Aug 2007
Pickard CE, Stewart AD, Hartley R, Lucock MD (1986) A rapid HPLC method for monitoring plasma levels of caffeine and theophylline using solid phase extraction columns. Ann Clin Biochem 23:440–446
Rasmussen BB, Jeppesen U, Gaist D, Brøsen K (1997) Griseofulvin and fluvoxamine interactions with the metabolism of theophylline. Ther Drug Monit 19:56–62
Rowland M, Matin S (1973) Kinetics of drug-drug interactions. J Pharmacokinet Biopharm 1:553–567
Tang C, Shou M, Rodrigues AD (2000) Substrate-dependent effect of acetonitrile on human liver microsomal cytochrome P450 2C9 (CYP2C9) activity. Drug Metab Dispos 28:567–572
Tassaneeyakul W, Birkett DJ, Veronese ME, McManus ME, Tukey RH, Quattrochi LC, Gelboin HV, Miners JO (1993) Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J Pharmacol Exp Ther 265:401–407
Werner U, Werner D, Rau T, Fromm MF, Hinz B, Brune K (2003) Celecoxib inhibits metabolism of cytochrome P450 2D6 substrate metoprolol in humans. Clin Pharmacol Ther 74:130–137
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karjalainen, M.J., Neuvonen, P.J. & Backman, J.T. Celecoxib is a CYP1A2 inhibitor in vitro but not in vivo. Eur J Clin Pharmacol 64, 511–519 (2008). https://doi.org/10.1007/s00228-007-0456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0456-4