Abstract
Objective
Zopiclone is a short acting hypnotic, which is metabolised by cytochrome P450 (CYP) 3A4 and 2C8 in vitro. We studied the possible effect of gemfibrozil, an inhibitor of CYP2C8, on the pharmacokinetics and pharmacodynamics of zopiclone.
Methods
In a randomised 2-phase crossover study, 10 healthy volunteers took 600 mg gemfibrozil or placebo orally twice daily for 3 days. On day 3, each ingested a 7.5 mg dose of zopiclone. Plasma concentrations and urinary excretion of zopiclone and its two primary metabolites, plasma gemfibrozil, and psychomotor performance were measured. The effects of CYP2C8, CYP2C9 and CYP3A4 inhibitors on the depletion of zopiclone (500 nM) were studied in vitro in human liver microsomes.
Results
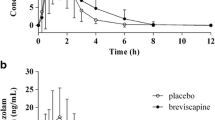
The pharmacokinetic variables of the parent zopiclone were not significantly affected by gemfibrozil. However, gemfibrozil raised the mean peak plasma concentration (Cmax) of N-oxide-zopiclone (1.6-fold; P<0.001) and that of N-desmethyl-zopiclone (1.2-fold; P<0.001). The mean area under the plasma concentration-time curve (\({\text{AUC}}_{{{\text{0}} - \infty }}\)) values of N-oxide-zopiclone and N-desmethyl-zopiclone were raised 2-fold (P<0.001) and 1.2-fold (P<0.01), respectively. The renal clearance of N-oxide-zopiclone was reduced by 48% by gemfibrozil (P<0.001). The pharmacodynamic effects of zopiclone, measured using psychometric tests, were not affected by gemfibrozil. In vitro, ketoconazole (1 μM) and itraconazole (8 μM) decreased the elimination rate of zopiclone enantiomers by about 65–95%, while montelukast (16 μM), gemfibrozil (200 μM) and sulfaphenazole (10 μM) had no appreciable effect.
Conclusions
Gemfibrozil does not increase the plasma concentrations of the parent zopiclone. Accordingly, CYP2C8 does not significantly metabolise zopiclone in vivo. However, as gemfibrozil raises the concentrations of two potentially active metabolites of zopiclone, slightly enhanced effects of zopiclone by gemfibrozil can not be excluded.



Similar content being viewed by others
References
Noble S, Langtry HD, Lamb HM (1998) Zopiclone. An update of its pharmacology, clinical efficacy and tolerability in the treatment of insomnia. Drugs 55:277–302
Gaillot J, Heusse D, Hougton GW, Marc Aurele J, Dreyfus JF (1983) Pharmacokinetics and metabolism of zopiclone. Pharmacology 27(Suppl 2):76–91
Goa KL, Heel RC (1986) Zopiclone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy as an hypnotic. Drugs 32:48–65
Carlson JN, Haskew R, Wacker J, Maisonneuve IM, Glick SD, Jerussi TP (2001) Sedative and anxiolytic effects of zopiclone's enantiomers and metabolite. Eur J Pharmacol 415:181–189
Fernandez C, Martin C, Gimenez F, Farinotti R (1995) Clinical pharmacokinetics of zopiclone. Clin Pharmacokinet 29:431–441
Becquemont L, Mouajjah S, Escaffre O, Beaune P, Funck-Brentano C, Jaillon P (1999) Cytochrome P-450 3A4 and 2C8 are involved in zopiclone metabolism. Drug Metab Dispos 27:1068–1073
Totah RA, Rettie AE (2005) Cytochrome P450 2C8: substrates, inhibitors, pharmacogenetics, and clinical relevance. Clin Pharmacol Ther 77:341–352
Aranko K, Luurila H, Backman JT, Neuvonen PJ, Olkkola KT (1994) The effect of erythromycin on the pharmacokinetics and pharmacodynamics of zopiclone. Br J Clin Pharmacol 38:363–367
Jalava KM, Olkkola KT, Neuvonen PJ (1996) Effect of itraconazole on the pharmacokinetics and pharmacodynamics of zopiclone. Eur J Clin Pharmacol 51:331–334
Villikka K, Kivistö KT, Lamberg TS, Kantola T, Neuvonen PJ (1997) Concentrations and effects of zopiclone are greatly reduced by rifampicin. Br J Clin Pharmacol 43:471–474
Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ (2002) Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther 72:685–691
Wang JS, Neuvonen M, Wen X, Backman JT, Neuvonen PJ (2002) Gemfibrozil inhibits CYP2C8-mediated cerivastatin metabolism in human liver microsomes. Drug Metab Dispos 30:1352–1356
Niemi M, Backman JT, Neuvonen M, Neuvonen PJ (2003) Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia 46:347–351
Jaakkola T, Backman JT, Neuvonen M, Neuvonen PJ (2005) Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics of pioglitazone. Clin Pharmacol Ther 77:404–414
Niemi M, Backman JT, Granfors M, Laitila J, Neuvonen M, Neuvonen PJ (2003) Gemfibrozil considerably increases the plasma concentrations of rosiglitazone. Diabetologia 46:1319–1323
Hengy H, Kölle EU (1985) Determination of gemfibrozil in plasma by high performance liquid chromatography. Arzneimittelforschung 35:1637–1639
Blaschke G, Hempel G, Müller WE (1993) Preparative and analytical separation of the zopiclone enantiomers and determination of their affinity to the benzodiazepine receptor binding site. Chirality 5:419–421
Shitara Y, Hirano M, Sato H, Sugiyama Y (2004) Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/OATP1B1:SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin: analysis of the mechanism of the clinically relevant drug-drug interaction between cerivastatin and gemfibrozil. J Pharmacol Exp Ther 311:228–236
Ogilvie BW, Zhang D, Li W, Rodrigues AD, Gipson AE, Holsapple J, Toren P, Parkinson A (2006) Glucuronidation converts gemfibrozil to a potent, metabolism-dependent inhibitor of CYP2C8: implications for drug-drug interactions. Drug Metab Dispos 34:191–197
Walsky RL, Obach RS, Gaman EA, Gleeson JP, Proctor WR (2005) Selective inhibition of human cytochrome P4502C8 by montelukast. Drug Metab Dispos 33:413–418
Wen X, Wang JS, Backman JT, Kivistö KT, Neuvonen PJ (2001) Gemfibrozil is a potent inhibitor of human cytochrome P450 2C9. Drug Metab Dispos 29:1359–1361
Baldwin SJ, Bloomer JC, Smith GJ, Ayrton AD, Clarke SE, Chenery RJ (1995) Ketoconazole and sulphaphenazole as the respective selective inhibitors of P4503A and 2C9. Xenobiotica 25:261–270
Fernandez C, Maradeix V, Gimenez F, Thuillier A, Farinotti R (1993) Pharmacokinetics of zopiclone and its enantiomers in Caucasian young healthy volunteers. Drug Metab Dispos 21:1125–1128
Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ (2003) Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin Pharmacol Ther 73:538–544
Acknowledgements
This study was supported by grants from the Helsinki University Central Hospital Research Fund, the National Technology Agency, and the Sigrid Jusélius Foundation, Finland. None of the authors has any financial or personal relationships that could be perceived as influencing the research described. The experiments comply with the current laws of Finland, and the study protocol was approved by the Ethics Committee for Studies in Healthy Subjects and Primary Care of the Hospital District of Helsinki and Uusimaa, and the Finnish National Agency for Medicines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tornio, A., Neuvonen, P.J. & Backman, J.T. The CYP2C8 inhibitor gemfibrozil does not increase the plasma concentrations of zopiclone. Eur J Clin Pharmacol 62, 645–651 (2006). https://doi.org/10.1007/s00228-006-0155-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0155-6