Abstract
Objective
To investigate the transfer of reboxetine into milk, the absolute and relative infant doses via milk and to assess plasma concentrations and adverse unwanted effects in the breastfed infant.
Methods
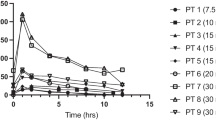
Multiple samples of blood and milk were obtained over a dose interval at steady-state from four women who were taking reboxetine for postnatal depression. Drug concentrations in plasma and milk were measured by high performance liquid chromatography and milk/plasma ratio (M/P), absolute infant dose and relative infant dose were estimated by standard methods. Their four, breastfed, infants were also examined clinically, and a blood sample was taken for drug analysis.
Results
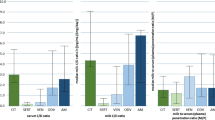
The median (range) dose taken by the women was 6 (4-10) mg/day. There was no significant difference in reboxetine concentration between paired fore-and hind-milk samples. The mean (95% CI) M/P was 0.06 (0.03, 0.09). Absolute infant dose was 1.7 (0.7, 2.4) μg/kg/day for reboxetine while the relative infant dose was 2.0% (1.3, 2.7%). Three of the infants met normal developmental milestones and no adverse effects were seen in any infant. The fourth infant had developmental problems that were not associated with the maternal reboxetine therapy. The concentrations of reboxetine in plasma from the four infants were <4 μg/l, 2.6 μg/l, 2.3 μg/l and 5 μg/l, respectively.
Conclusion
The study suggests that reboxetine use by lactating women is safe for the breastfed infant. Nevertheless, our study had only four mother/baby pairs, and each decision to breastfeed should always be made on the basis of an individual risk/benefit analysis.

Similar content being viewed by others
References
O’Hara MW, Zekoski EM, Philipps LH, Wright EJ (1990) Controlled prospective study of postpartum mood disorders: comparison of childbearing and nonchildbearing women. J Abnorm Psychol 99:3–15
Sinclair D, Murray L (1998) Effects of postnatal depression on children’s adjustment to school. Teacher’s reports. Brit J Psychiatry 172:58–63
Zekoski EM, O’Hara MW, Wills KE (1987) The effects of maternal mood on mother-infant interaction. J Abnorm Child Psychol 15:361–378
Lee CM, Gotlib IH (1991) Adjustment of children of depressed mothers: a 10-month follow-up. J Abnorm Psychol 100:473–477
Murray L, Fiori-Cowley A, Hooper R, Cooper P (1996) The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev 67:2512–2526
Anonymous (2005) Edronax, MIMS Abbreviated Prescribing Information. Version 5.00.0248 Edition. MediMedia Australia, St Leonards, Australia
Ohman D, Cherma MD, Norlander B, Bengtsson F (2003) Determination of serum reboxetine enantiomers in patients on chronic medication with racemic reboxetine. Ther Drug Monit 25:174–182
Burrows GD, Maguire KP, Norman TR (1998) Antidepressant efficacy and tolerability of the selective norepinephrine reuptake inhibitor reboxetine: a review. J Clin Psychiatry 59(Suppl) 14:4–7
Mucci M (1997) Reboxetine: a review of antidepressant tolerability. J Psychopharmacol 11:S33–S37
Mann JJ (2005) The medical management of depression. New Engl J Med 353:1819–1834
National Centre for Health Statistics, Clinical growth charts. Available at: http://www.cdc.gov/nchs/data/nhanes/growthcharts/set1clinical/Cj41c017.pdf http://www.cdc.gov/nchs/data/nhanes/growthcharts/set1clinical/Cj41c018.pdf Accessed: 24-1-2006
Frankenburg WK, Dodds JB (1967) The Denver development screening test. J Pediatr 71:181–191
Rossiter EJ (1993) The use of developmental screening and assessment instruments by paediatricians in Australia. J Paediatr Child Health 29:357–359
Rampono J, Kristensen JH, Hackett LP, Paech M, Kohan R, Ilett KF (2000) Citalopram and demethylcitalopram in human milk; distribution, excretion and effects in breast fed infants. Br J Clin Pharmacol 50:263–268
Kristensen JH, Ilett KF, Dusci LJ, Hackett LP, Yapp P, Wojnar-Horton RE, Roberts MJ, Paech M (1998) Distribution and excretion of sertraline and N-desmethylsertraline in human milk. Br J Clin Pharmacol 45:453–457
Begg EJ, Duffull SB, Hackett LP, Ilett KF (2002) Studying drugs in human milk: time to unify the approach. J Hum Lact 18:319–328
Bennett PN (1996) Use of the monographs on drugs. In: Bennett PN (ed) Drugs and human lactation, 2nd edn. Elsevier, Amsterdam, pp 67–74
Hale TW, Ilett KF (2002) Drug therapy and breastfeeding. From theory to clinical practice, 1st edn. Parthenon, London, pp 1–94
Lucas A, Gibbs JA, Lyster RL, Baum JD (1978) Creamatocrit: simple clinical technique for estimating fat concentration and energy value of human milk. Br Med J (Clin Res Edn) 1:1018–1020
Kim J, Riggs KW, Misri S, Kent N, Oberlander TF, Grunau RE, Fitzgerald C, Rurak DW (2006) Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol 61:155–163
Begg EJ, Atkinson HC, Duffull SB (1992) Prospective evaluation of a model for the prediction of milk:plasma drug concentrations from physicochemical characteristics. Br J Clin Pharmacol 33:501–505
Rampono J, Hackett LP, Kristensen JH, Kohan R, Page-Sharp M, Ilett KF (2006) Transfer of escitalopram and its metabolite demethylescitalopram into breastmilk. Br J Clin Pharmacol (in press)
Edwards DM, Pellizzoni C, Breuel HP, Berardi A, Castelli MG, Frigerio E, Poggesi I, Rocchetti M, Dubini A, Strolin Benedetti M (1995) Pharmacokinetics of reboxetine in healthy volunteers. Single oral doses, linearity and plasma protein binding. Biopharm Drug Dispos 16:443–460
McNamara PJ, Alcorn J (2002) Protein binding predictions in infants. AAPS Pharm Sci 4:1–7
Acknowledgements
This work was supported by a grant from Pfizer Australia. We gratefully acknowledge the assistance of Deborah Oosterbaan and Mary Wallbank with sample collection on the study days.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hackett, L.P., Ilett, K.F., Rampono, J. et al. Transfer of reboxetine into breastmilk, its plasma concentrations and lack of adverse effects in the breastfed infant. Eur J Clin Pharmacol 62, 633–638 (2006). https://doi.org/10.1007/s00228-006-0140-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0140-0