Abstract
Objective
The aim of the present study was to evaluate the possible influence of atorvastatin on the pharmacokinetics of cyclosporine (INN ciclosporin) and its main metabolites, AM1 and AM9, in renal transplant recipients.
Methods
Whole blood samples from 18 renal transplanted patients on cyclosporine-based immunosuppressive therapy were collected prior to and after 4 weeks of treatment with atorvastatin (10 mg/day) and analysed with regard to both cyclosporine and its main metabolites, AM1 and AM9, using a specific chromatographic method with ultraviolet detection.
Results
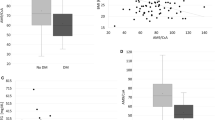
On average, AUC0-12 [area under the whole blood concentration versus time curve in the dosing interval (0–12 h)] of cyclosporine was 5% (−16, 5) (90% confidence interval) lower upon co-administration with atorvastatin. No statistically significant changes in any of the calculated pharmacokinetic variables [AUC0-12, maximum whole blood concentration (Cmax), whole blood concentration 12 h post dose (C12), time to Cmax (tmax), terminal half-life (t1/2)] for cyclosporine or the two metabolites, AM1 and AM9, upon atorvastatin treatment were observed. On average, atorvastatin did not affect the ratio between the CYP3A4-mediated metabolite AM9 and cyclosporine, suggesting that atorvastatin does not affect the CYP3A4 metabolism of cyclosporine to any significant extent. However, the influence of atorvastatin on the ratio between AM9 and cyclosporine showed large interindividual variability.
Conclusion
The results of this study indicate that atorvastatin does not, on average, affect cyclosporine pharmacokinetics in renal transplant recipients.

Similar content being viewed by others

References
Asberg A (2003) Interactions between cyclosporin and lipid-lowering drugs: implications for organ transplant recipients. Drugs 63:367–378
Asberg A, Hartmann A, Fjeldså E, Bergan S, Holdaas H (2001) Bilateral pharmacokinetic interaction between cyclosporine A and atorvastatin in renal transplant recipients. Am J Transplant 382–386
Gomez DY, Wacher VJ, Tomlanovich SJ, Hebert MF, Benet LZ (1995) The effects of ketoconazole on the intestinal metabolism and bioavailability of cyclosporine. Clin Pharmacol Ther 58:15–19
Hermann M, Asberg A, Christensen H, Holdaas H, Hartmann A, Reubsaet JL (2004) Substantially elevated levels of atorvastatin and metabolites in cyclosporine-treated renal transplant recipients. Clin Pharmacol Ther 76:388–391
Hermann M, Asberg A, Reubsaet JL, Sather S, Berg KJ, Christensen H (2002) Intake of grapefruit juice alters the metabolic pattern of cyclosporin A in renal transplant recipients. Int J Clin Pharmacol Ther 40:451–456
Hermann M, Christensen H, Reubsaet JL (2002) Evaluation of essential parameters in the chromatographic determination of cyclosporin A and metabolites using a D-optimal design. J Pharm Biomed Anal 30:1263–1276
Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG (1999) A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem 274:37161–37168
Kantola T, Kivisto K, Neuvonen P (1998) Effect of itraconazole on the pharmacokinetics of atorvastatin. Clin Pharmacol Ther 64:58–65
Mahalati K, Belitsky P, Sketris I, West K, Panek R (1999) Neoral monitoring by simplified sparse sampling area under the concentration–time curve: its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantation. Transplantation 68:55–62
Omar MA, Wilson JP (2002) FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother 36:288–295
Renders L, Mayer-Kadner I, Koch C, Scharffe S, Burkhardt K, Veelken R, Schmieder RE, Hauser IA (2001) Efficacy and drug interactions of the new HMG-CoA reductase inhibitors cerivastatin and atorvastatin in CsA-treated renal transplant recipients. Nephrol Dial Transplant 16:141–146
Romero R, Calvino J, Rodriguez J, Sanchez-Guisande D (2000) Short-term effect of atorvastatin in hypercholesterolaemic renal-transplant patients unresponsive to other statins. Nephrol Dial Transplant 15:1446–1449
Steinijans VW, Hartmann M, Huber R, Radtke HW (1991) Lack of pharmacokinetic interaction as an equivalence problem. Int J Clin Pharmacol Ther Toxicol 29:323–328
Taylor PJ, Kubler PA, Lynch SV, Allen J, Butler M, Pillans PI (2004) Effect of atorvastatin on cyclosporine pharmacokinetics in liver transplant recipients. Ann Pharmacother 38:205–208. Epub 2003 Dec 2019
Winkler M, Schumann G, Petersen D, Oellerich M, Wonigeit K (1992) Monoclonal fluorescence polarization immunoassay evaluated for monitoring cyclosporine in whole blood after kidney, heart and liver transplantation. Clin Chem 38:123–126
Wu X, Whitfield LR, Stewart BH (2000) Atorvastatin transport in the Caco-2 cell model: contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharm Res 17:209–215
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermann, M., Åsberg, A., Christensen, H. et al. Atorvastatin does not affect the pharmacokinetics of cyclosporine in renal transplant recipients. Eur J Clin Pharmacol 61, 59–62 (2005). https://doi.org/10.1007/s00228-004-0874-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0874-5