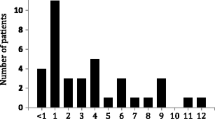
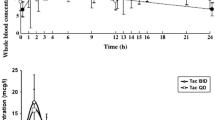
Abstract
Cyclosporine (CsA) is a critical-dose drug for which a minor change in absorption can have important clinical implications. Generic formulations of CsA are becoming more widely available, but standard criteria for bioequivalence require only that a single study in healthy volunteers demonstrate that mean pharmacokinetic parameters fall within 80–125% of the mean values for Neoral, the reference formulation of CsA. However, CsA absorption is known to differ between healthy volunteers and transplant patients and between different types of transplant patients, such that standard bioequivalence testing may be inadequate to ensure interchangeability of CsA formulations in all patients. The limited available clinical evidence has shown that stable renal transplant patients receiving Neoral have a significant reduction in mean CsA trough level after transfer to the Cicloral formulation. Mean pharmacokinetic values have been reported as equivalent following transfer to Gengraft in one study, but mean CsA trough fell and mean serum creatinine rose significantly in a separate trial. The only clinical outcomes data available are from a retrospective study of de novo renal transplant patients, which reported a significantly higher incidence of biopsy-proven acute rejection in patents receiving Gengraf versus Neoral (39% versus 25%, P<0.05). Until robust clinical data demonstrate that different formulations of CsA are interchangeable, it is advisable to prescribe CsA by brand, and any transfer to a different CsA formulation should be undertaken with close supervision and only at the direction of the transplant physician.



Similar content being viewed by others
References
Pollard S, Nashan B, Johnston A et al (2003) A pharmacokinetic and clinical review of the potential clinical impact of using different formulations of cyclosporin A. Clin Ther 25:1654–1669
Kovarik JM, Mueller EA, van Bree JB et al (1994) Cyclosporine pharmacokinetics and variability from a microemulsion formulation—a multicenter investigation in kidney transplant patients. Transplantation 58:658–663
Lindholm A, Kahan BD (1993) Influence of cyclosporine pharmacokinetics, trough concentrations, and AUC monitoring on outcome after kidney transplantation. Clin Pharmacol Ther 54:205–218
Kahan BD (1996) Variable oral absorption of cyclosporine. A biopharmaceutical risk factor for chronic renal allograft rejection. Transplantation 62:599–606
Humar A, Kerr S, Gillingham KJ, Matas AG (1999) Features of acute rejection that increase risk for chronic rejection. Transplantation 68:1200–1203
Basadonna GP, Matas AJ, Gillingham KJ et al (1993) Early versus later acute renal allograft rejection: impact on chronic rejection. Transplantation 55:993–995
Hariharan S, McBride MA, Cherikh SW et al (2002) Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 62:311–318
Kahan BD, Dunn J, Fitts C et al (1995) Reduced inter- and intrasubject variability in cyclosporine pharmacokinetics in renal transplant recipients treated with a microemulsion formulation in conjunction with fasting, low-fat meals, or high-fat meals. Transplantation 59:505–511
Holt DW, Mueller EA, Kovarik JM, van Bree JB, Richard F, Kutz K (1995) Sandimmun Neoral pharmacokinetics: impact of the new oral formulation. Transplant Proc 27:1434–1437
Committee for Proprietary Medicinal Products (CPMP) (2001) Notes for guidance on the investigation of bioavailability and bioequivalence. London,http://www.emea.eu.int/pdfs/human/ewp/140198en.pdf Accessed 22 October 2003
Food and Drug Administration Center for Drug Evaluation and Research (2001) Approved drug products with therapeutic equivalence evaluations prescribing information.http://www.fda.gov/cder/guidance/4964dft.pdf Accessed 22 October 2003
Johnston A, Keown PA, Holt DW (1997) Simple bioequivalence criteria: are they relevant to critical dose drugs? Experience gained from cyclosporine. Ther Drug Monit 19:375–378
Mueller EA, Kovarik JM, van Bree JB, Tetzloff W, Grevel J, Kutz K (1994) Improved dose linearity of cyclosporine pharmacokinetics from a microemulsion formulation. Pharm Res 11:301–304
Cooney GF, Jeevanandam V, Choudhury S, Feutren G, Mueller EA, Eisen H (1998) Comparative bioavailability of Neoral and Sandimmune in cardiac transplant recipients over 1 year. Transplantation 30:1892–1894
Freeman D, Grant D, Levy G et al (1995) Pharmacokinetics of a new oral formulation of cyclosporine in liver transplant recipients. Ther Drug Monit 17:213–216
Cooney GF, Habucky K, Hoppu K (1997) Cyclosporin pharmacokinetics in paediatric transplant recipients. Clin Pharmacokinet 32:481–495
Dunn S, Cooney G, Sommerauer J et al (1997) Pharmacokinetics of an oral solution of the microemulsion formulation of cyclosporine in maintenance pediatric liver transplant recipients. Transplantation 63:1762–1767
Schroeder TJ, Hariharan S, First MR (1995) Variations in bioavailability of cyclosporine and relationship to clinical outcome in renal transplant subpopulations. Transplant Proc 27:837–839
Lindholm A, Welsh M, Rutzky L et al (1993) The adverse impact of high cyclosporine: clearance rates on the incidences of acute rejection and graft loss. Transplantation 55:985–993
Cooney GF, Fiel SB, Shaw LM et al (1990) Cyclosporine bioavailability in heart-lung transplant candidates with cystic fibrosis. Transplantation 49:821–823
Tan KKC, Hue KL, Strickland SE et al (1990) Altered pharmacokinetics of cyclosporin in heart-lung transplant recipients with cystic fibrosis. Ther Drug Monit 12:520–524
Federal Register (2000) December 4, 2000; 65 (233). Available athttp://www.fda.gov/OHRMS/DOCKETS/98fr/120400a.htm Accessed 30 September 2003
Nashan B, Bleck J, Wonigeit K et al (1988) Effect of the application form of cyclosporine on blood levels: comparison of the oral solution and capsules. Transplant Proc 20:637–639
Curtis JJ, Barbeito R, Pirsch J, Lewis RM, Van Buren DH, Choudhury S (1999) Differences in bioavailability between oral cyclosporine formulations in maintenance renal transplant patients. Am J Kidney Dis 34:869–874
Koehler J, Kuehnel T, Kees F et al (2002) Comparison of bioavailability and metabolism with two commercial formulations of cyclosporine A in rats. Drug Metab Dispos 30:658–662
Wandel C, Kim RB, Stein CM (2003) “Inactive” excipients such as cremophor can affect in vivo drug disposition. Clin Pharmacol Ther 73:394–396
Based on Study Report number ANA-97–132 submitted by Eon Labs Manufacturing, Inc., Laurelton, NY (USA) Department of Public Health
Neoral Prescribing Information, Novartis Pharma AG, Basel, Switzerland
Based on Study Report number ANA-97–133 submitted by Eon Labs Manufacturing, Inc., Laurelton, NY (USA) to the Illinois (USA) Department of Public Health
Based on Biostudy Synopsis M97–686 submitted by Abbott Laboratories, Abbott Park, IL (USA) to the New Jersey (USA) Department of Health and Public Services, Drug Utilization Review Council
Kunz R, Hecker J, Lorenz R et al (2002) Analysis of the dose/blood level ratio of ciclosporin (CyA) in stable kidney transplant patients with the administration of different CyA formulations [abstract]. 11th Annual Conference of the German Transplantation Society, 24–26 October 2002, Hannover
Roza A, Tomlanovich S, Merion R et al (2002) Conversion of stable renal allograft recipients to a bioequivalent cyclosporine formulation. Transplantation 74:1013–1017
Qazi YA, Forrest A, Tornatore K et al (2002) The clinical and economic impact of 1:1 conversion from Neoral to Gengraf (abstract). ASN 2002, SA-P0505
Shah MB, Martin JE, Schroeder TJ et al (1999) The evaluation of the safety and tolerability of two formulations of cyclosporine: Neoral and Sandimmune. A meta-analysis. Transplantation 67:1411–1417
Meier-Kriesche H-U, Kaplan B (2002) Cyclosporine microemulsion and tacrolimus are associated with decreased chronic allograft failure and improved long-term graft survival as compared with Sandimmun. Am J Transplant 2:100–104
Taber DJ, Baillie GM, Ashcraft E et al (2003) Bioequivalence between cyclosporine microemulsion formulations may not translate into equivalent clinical outcomes (abstract). American Transplant Congress 30 May–1 June 2003, Washington DC, Abstract 1211
British National Formulary Issue 46, September 2003,http://www.bnf.org Accessed 1 October 2003
Durlik M, Rauch C, Thyroff-Friesinger U, Streu H, Paczek L (2003) Comparison of peak and trough level monitoring of cyclosporine treatment using two modern cyclosporine preparations. Transplant Proc 35:1304–1307
http://www.hc-sc.gc.ca/hpfb-dgpsa/tpd-dpt/2001–03–22_eac_bb_e.pdf Accessed 24 November 2003
Levy G (1995) The clay feet of bioequivalence testing. J Pharm Pharmacol 47:975–977
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnston, A., Belitsky, P., Frei, U. et al. Potential clinical implications of substitution of generic cyclosporine formulations for cyclosporine microemulsion (Neoral) in transplant recipients. Eur J Clin Pharmacol 60, 389–395 (2004). https://doi.org/10.1007/s00228-004-0774-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0774-8