Abstract
Objective
To compare potency and efficacy of dexamethasone (DEXA) and prednisolone (PRED) in assumed equipotent doses in combination with endogenous cortisol, using lymphocyte counts, plasma osteocalcin (OC), and eosinophilic cationic protein (ECP) as effect variables and to evaluate potential differences between healthy subjects and asthmatic patients.
Methods
Eight healthy subjects and six asthmatic patients who had stopped taking their regular inhaled glucocorticosteroid treatment (ICS) for 1 week, were given an IV bolus of DEXA and PRED in assumed equipotent doses of 2.0 mg and 12.5 mg, respectively, on separate occasions, in combination with subcutaneously injected granulocyte–colony-stimulating factor (G-CSF) as a stimulant for ECP production. Plasma levels of DEXA, PRED, cortisol and effect variables were determined over 25 h and pharmacokinetic–pharmacodynamic (PK–PD) modelling was performed.
Results
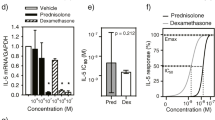
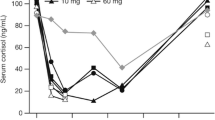
Baseline cortisol concentration was lower in patients than in healthy subjects. Both of the exogenous glucocorticoids (GCs) diminished cortisol production. In the healthy subjects, the cortisol production remained suppressed for the full duration of the study day after DEXA but not after PRED. In the asthmatic patients though, the reappearance of the endogenous production of cortisol was seen after both DEXA and PRED. The Emax values for lymphocyte counts and OC showed that cortisol acted as partial, and DEXA and PRED as full agonists. The observed responses of DEXA and PRED suppressing cortisol, OC and lymphocyte counts were all of the same relative order of magnitude, in accordance with the estimated PD parameters. However, cortisol was estimated to have very little effect on ECP and modelling further predicted that DEXA and PRED were only partial agonists for this effect, without a difference between healthy and asthmatic subjects. Yet, in healthy subjects, the area under the concentration–time curves (AUCs) indicated unexpectedly that ECP was only suppressed after PRED and not after DEXA, while in patients it was suppressed after both GCs. The rank order of potency on lymphocyte counts, OC and ECP was DEXA>PRED>cortisol, although the different relative potencies of the three GCs involved were not the same for all of the three effect variables and differences were also found between healthy and asthmatic subjects.
Conclusion
PK–PD modelling studies of GCs demonstrated not only differences in potency of DEXA and PRED on the measured systemic markers, but also different potencies per target tissue and differences between healthy and asthmatic men. The effects caused by the achieved blood concentrations of DEXA and PRED, expressed as AUCs of the effect variables, were in accordance with their respective Emax values in case of the lymphocytes and OC but not for ECP.




Similar content being viewed by others
References
Marble A, Selenkow HA, Rose LI, Dluhy RG, Williams GH (1980) Endocrine diseases. In: Avery GS (ed) Drug treatment, 2nd edn. Churchill Livingstone, Edinburgh and London, p 530
McKenzie AW, Stoghton RB (1962) Method for comparing percitaneous absorption of steroids. Arch Dermatol 86:608-610
Sommer A, Veraart J, Neumann M, Kessels A (1998) Evaluation of the vasoconstrictive effects of topical steroids Doppler-perfusion-imaging. Acta Derm Venereol 78:15–18
Noon JP, Evans CE, Haynes WG, Webb DJ, Walker BR (1996) A comparison of techniques to assess skin blanching following the topical application of glucocorticoids. Br J Dermatol 134:837–842
Langhoff E, Ladefoged J (1983) Relative immunosuppressive potency of various corticosteroids measured in vitro. Eur J Clin Pharmacol 25:459–462
Mullol J, Xaubet A, Lopez E, Roca–Ferrer J, Picado C (1995) Comparative study of the effects of different glucocorticosteroids on eosinophil survival primed by cultured epithelial cell supernatants obtained from nasal mucosa and nasal polyps. Thorax 50:270–274
Snijdewint FG, Kapsenberg ML, Wauben–Penris PJ, Bos JD (1995) Corticosteroids class dependently inhibit in vitro Th1- and Th2-type cytokine production. Immunopharmacology 29:93–101
Derks MGM, Dubois EFL, Koopmans RP, Boxtel van CJ (1999) Effect of hydrocortisone on plasma tyrosine concentration and lymphocyte counts in healthy volunteers. Clin Drug Invest 18:390–401
Ekenstam E, Stålenheim G, Hällgren R (1988) The acute effect of high dose corticosteroid treatment on serum osteocalcin. Metabolism 37:141–144
Godschalk MF, Downs RW (1988) Effect of short-term glucocorticoids on serum osteocalcin in healthy young men. J Bone Miner Res 3:113–115
Bijlsma JWJ, Lems WF, Gerrits MI, Jacobs JWG, van Vugt RM, van Rijn HJM (1995) Effect of high-dose corticosteroid pulse therapy on (markers of) bone formation and resorption in rheumatoid arthritis (abstract). Calcif Tissue Int 56:500
Gerrits MI, Lems WF, Bijlsma JWJ, Thijssen JHH, van Rijn HJM (1996) Botmetabolisme een overzicht van Klinisch–chemische markers. Ned Tijdschr Klin Chem 21:184–192
Khosla S, Kleerekoper M (1999) Biochemical markers of bone turnover. In: Favus MJ, Goldring SR, Christakos S (eds) Primer on the bone diseases and disorders of mineral metabolism 4th edn. Lippincott, Williams and Wilkins, Philadelphia, pp 128–134
Epstein S (1988) Serum and urinary markers of bone remodeling: assessment of bone turnover. Endocr Rev 9:437–449
Lian JB, Gundberg CM (1988) Osteocalcin: biochemical considerations and clinical applications. Clin Orthopaed Relat Res 226:267–291
Szulc P, Seeman E, Delmas PD (2000) Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int 11:281–294
Wald J, Jusko W (1992) Corticosteroid pharmacodynamic modelling: osteocalcin suppression by prednisolone. Pharm Res 9:1096–1098
Nielsen H, Charles P, Moskilde L (1988) The effect of single oral doses of prednisolone in circadian rhythm of serum osteocalcin in normal subjects. J Clin Endocrinol Metab 67:1025–1030
Nielsen HK, Laurberg P, Brixen K, Mosekilde L (1991) Relationship between diurnal variations in serum osteocalcin, cortisol, parathyroid hormone, and ionized calcium in normal individuals. Acta Endocrinol 124:391–398
Nielsen HK, Brixen K, Kassem M, Charles P, Moskilde L (1992) Inhibition of the morning cortisol peak abolishes the expected morning decrease in serum osteocalcin in normal males: evidence of a controlling effect of serum cortisol on the circadian rhythm in serum osteocalcin. J Clin Endocrinol Metab 74:1410–1414
Heshmati HM, Riggs BL, Burritt MF, Mc Allister CA, Wollan PC, Khosla S (1998) Effects of the circadian variation in serum cortisol on markers of bone turnover and calcium homeostasis in normal postmenopausal women. J Clin Endocrinol Metab 83:751–756
Dubois EFL, Derks MGM, Zwinderman AH, Dekhuijzen PNR, van Boxtel CJ, Schweitzer DH (2003) Distinct actions of prednisolone and dexamethasone towards osteocalcin and eosinophilic cationic protein in assumed clinically equivalent doses: a study in healthy men. Eur J Clin Pharmacol 58:733–737
Denburg JA (1997) Effects of corticosteroids on basophils and mast cells. In: Schleimer RP, Busse WW, O’Byrne PM (eds) Lung biology in health and disease. Marcel Dekker, New York, pp 259–277
Wever AM, Wever Hess J, Hermans J (1997) The use of serum eosinophil cationic protein (ECP) in the management of steroid therapy in chronic asthma. Clin Exp Allergy 27:519–529
Karawajczyk M, Höglund M, Ericsson J, Venge P (1997) Administration of G-CSF to healthy subjects: the effects on eosinophil counts and mobilization of eosinophil granule proteins. Br J Haematol 96:259–265
van Boxtel CJ, Oosterhuis B (1992) Pharmacokinetic–pharmacodynamic modeling of corticosteroids. In: van Boxtel CJ, Holford NHG, Danhof M (eds) The in vivo study of drug action. Elsevier, Amsterdam, pp 357–377
Leuppi JD, Salome CM, Jenkins CR, Anderson SD, Xuan W, Marks GB, Koskela H, Brannan JD, Freed R, Andersson M, Chan HK, Woolcock AJ (2001) Predictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroids. Am J Respir Crit Care Med 163:406–412
Gibson PG, Wong BJ, Hepperle MJ, Kline PA, Girgis-Gabardo A, Guyatt G, Dolovich J, Denburg JA, Ramsdale EH, Hargreave FE (1992) A research method to induce and examine a mild exacerbation of asthma by withdrawal of inhaled corticosteroid. Clin Exp Allergy 22:525–532
Rose JQ, Jusko WJ (1979) Corticosteroid analysis in biological fluids by high-performance liquid chromatography. J Chromatogr 162:273–280
Masters PW, Jones RG, Purves DA, Cooper EH, Cooney JM (1994) Commercial essays for serum osteocalcin give clinically discordant results. Clin Chem 40:358–363
Anonymous (1986) Micromath, Inc., Scientist, ver 2.0. Salt MicroMath Scientific Software, Lake City
Gibaldi M, Perrier D (1975) Pharmacokinetics. Marcel Dekker, New York
Braat MC, Oosterhuis B, Koopmans RP, Meewis JM, van Boxtel CJ (1992) Kinetic–dynamic modeling of lymphocytopenia induced by the combined action of dexamethasone and hydrocortisone in humans, after inhalation and intravenous administration of dexamethasone. J Pharmacol Exp Ther 262:509–515
Holford NH, Sheiner LB (1982) Kinetics of pharmacologic response. Pharmacol Ther 16:143–166
Holford NH, Sheiner LB (1981) Pharmacokinetic and pharmacodynamic modeling in vivo. CRC Crit Rev Bioeng 5:273–322
Koopmans RP, Braat MC, Oosterhuis B, van Boxtel CJ (1992) Time-dependent effects of dexamethasone administration on the suppression of plasma hydrocortisone, assessed with a pharmacokinetic model. J Pharmacol Exp Ther 262:503–508
Aris RM, Stephens AR, Ontjes DA, Denene Blackwood A, Lark RK, Hensler MB, Neuringer IP, Lester GE (2000) Adverse alterations in bone metabolism are associated with lung infection in adults with cystic fibrosis. Am J Respir Crit Care Med 162:1674–1678
Cortet B, Guyot MH, Solau E, Pigny P, Dumoulin F, Flipo RM, Marchandise X, Delcambre B (2000) Factors influencing bone loss in rheumatoid arthritis: a longitudinal study. Clin Exp Rheumatol 18:683–690
Oosterhuis B, ten Berge RJ, Sauerwein HP, Endert E, Schellekens PT, van Boxtel CJ (1984) Pharmacokinetic–pharmacodynamic modeling of prednisolone-induced lymphocytopenia in man. J Pharmacol Exp Ther 229:539–546
Futijaka M, Kawaguchi H, Kato Y, Sakura N, Ueda K, Abe Y (2001) Significance of eosinophil cationic protein/eosinophil count ratio in asthmatic patients: its relationship to disease severity. Ann Allergy Immunol 86:323–329
Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG (2002) Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J 19:68–75
Malo JL, Cartier A, Ghezzo H, Mark S, Brown J, Laviolette M, Boulet LP (1999) Skin bruising, adrenal function and markers of bone metabolism in asthmatics using inhaled beclomethasone and fluticasone. Eur Respir J 13:993–998
Lipworth BJ (1999) Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med 159:941–55
Acknowledgements
We are indebted to Els Portier and Ineke Bosman, laboratory technicians, for their excellent support and the processing of the samples, to Esther Röder, MD, for her clinical support and to the commission “WOM” of the Hospital Reinier de Graaf Groep for their supportive grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubois, E.F.L., Derks, M.G.M., Schweitzer, D.H. et al. Pharmacokinetic/pharmacodynamic modelling of effects of dexamethasone and prednisolone in combination with endogenous cortisol on lymphocyte counts and systemic markers of bone turn over and inflammation in healthy and asthmatic men. Eur J Clin Pharmacol 60, 315–328 (2004). https://doi.org/10.1007/s00228-004-0738-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0738-z