Abstract
Objective
To evaluate the long-term effect of microcrystalline chitosan (MCC) on plasma lipids, especially the concentration of low-density lipoprotein (LDL) cholesterol, in subjects with a moderately increased concentration of plasma total cholesterol.
Methods
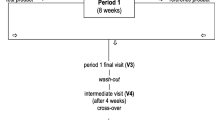
A total of 130 middle-aged men and women without severe disease and with a total cholesterol of 4.8–6.8 mmol/l and triglycerides below 3.0 mmol/l were randomised into two treatment groups. At the beginning of the 10-month trial, all participants received placebo 1.2 g twice daily during a 1-month run-in period. Subsequently, group 1 first received 1.2 g placebo twice daily for 3 months and then 1.2 g MCC twice daily for 3 months. Correspondingly, group 2 received 1.2 g MCC twice daily during the first and 1.2 g placebo twice daily during the second 3-month period. During the final 3-month follow-up period, both groups received MCC. Altogether, 83 participants completed the study.
Results
No difference was detected in the change in the LDL-cholesterol concentration between the treatments during the crossover trial (P=0.98 for interaction between time period and treatment group, repeated-measures analysis of variance for crossover design). In an otherwise similar analysis, no differences were detected between the treatments in the concentrations of total cholesterol, high-density lipoprotein cholesterol, triglycerides and glucose.
Conclusions
Treatment with MCC had no effect on the concentrations of plasma lipids or glucose in healthy middle-aged men and women with moderately increased plasma cholesterol concentrations.

Similar content being viewed by others
References
Ylitalo R, Lehtinen S, Wuolijoki E, Ylitalo P, Lehtimaki T (2002) Cholesterol-lowering properties and safety of chitosan. Arzneim Forsch 52:1–7
Jing SB, Li L, Ji D, Takiguchi Y, Yamaguchi T (1997) Effect of chitosan on renal function in patients with chronic renal failure. J Pharm Pharmacol 49:721–723
Wuolijoki E, Hirvela T, Ylitalo P (1999) Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods Find Exp Clin Pharmacol 2:357–361
Gallaher DD, Gallaher CM, Mahrt GJ, Carr TP, Hollingshead CH, Hesslink R Jr. et al (2002) A glucomannan and chitosan fiber supplement decreases plasma cholesterol and increases cholesterol excretion in overweight normocholesterolemic humans. J Am Coll Nutr 21:428–433
Pittler MH, Abbot NC, Harkness EF, Ernst E (1999) Randomized, double-blind trial of chitosan for body weight reduction. Eur J Clin Nutr 53:379–381
Ho SC, Tai ES, Eng PH, Tan CE, Fok AC (2001) In the absence of dietary surveillance, chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese Asian subjects. Singapore Med J 42:6–10
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Schaefer EJ (2002) Lipoproteins, nutrition, and heart disease. Am J Clin Nutr 75:191–212
Shahar DR, Yerushalmi N, Lubin F, Froom P, Shahar A, Kristal-Boneh E (2001) Seasonal variations in dietary intake affect the consistency of dietary assessment. Eur J Epidemiol 17:129–133
Brown WV (2001) Therapies on the horizon for cholesterol reduction. Clin Cardiol 24[Suppl]:III24–III27
Acknowledgements
We thank research nurse Saija Koivisto for skilful management of the examination visits and careful handling of the case report forms. The manufacturer of the study product and main sponsor of the study was Novasso Oy. Saara Metso was supported by a fellowship from the Emil Aaltonen Foundation. Terho Lehtimäki was funded by the Elli and Elvi Oksanen Fund of the Pirkanmaa Fund under the auspices of the Finnish Cultural Foundation and the Medical Research Fund of Tampere University Hospital (Tampere, Finland).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Metso, S., Ylitalo, R., Nikkilä, M. et al. The effect of long-term microcrystalline chitosan therapy on plasma lipids and glucose concentrations in subjects with increased plasma total cholesterol: a randomised placebo-controlled double-blind crossover trial in healthy men and women. Eur J Clin Pharmacol 59, 741–746 (2003). https://doi.org/10.1007/s00228-003-0691-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0691-2