Abstract
Objective
In vitro studies with human liver microsomes have suggested that the oxidative conversion of mexiletine (MX) to its metabolites is catalyzed by CYP2D6 and is significantly impaired in microsomes with the CYP2D6*10/*10 genotype. Therefore, we examined the influence of the CYP2D6*10 allele on MX pharmacokinetics in Japanese subjects.
Methods
Subjects with CYP2D6*1/*1 (group*1/*1; n=5), CYP2D6*10/*10 (group*10/*10; n=6) and CYP2D6*5/*10 (group*5/*10; n=4) genotypes received a single 200-mg dose of MX. Plasma and urinary levels of MX and its metabolites (p-hydroxymexiletine (PHM), hydroxymethylmexiletine (HMM) and N-hydroxymexiletine (NHM)) were determined by means of high-performance liquid chromatography.
Results
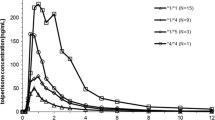
Mean area under the concentration–time curve (AUC) and t1/2 of MX were significantly (P<0.05) higher in the CYP2D6*10/*5 group (AUC 11.23±3.05 µg·h/ml; t1/2 15.5±3.2 h) than in the CYP2D6*1/*1 (AUC 5.53±1.01 µg·h/ml; t1/2 8.1±1.6 h) and CYP2D6*10/*10 (AUC 7.32±2.36 µg·h/ml; t1/2 10.8±2.8 h) groups, but there was no significant difference between the CYP2D6*1/*1 and CYP2D6*10/*10 groups. The maximum plasma concentration of MX was not significantly different among the three groups. The values of urinary excretion of PHM and HMM in the CYP2D6*1/*1 group were significantly (P<0.05) higher than those in the CYP2D6*10/*10 and CYP2D6*5/*10 groups, but there was no significant difference in that of NHM among the three groups. Clearance of MX in the CYP2D6*5/*10 subjects was comparable to that in the poor metabolizers described previously.
Conclusion
The present findings demonstrated that carriers of the CYP2D6*10 allele showed a decreased clearance of MX. Subjects with CYP2D6*5/*10 showed significantly (P<0.05) increased plasma levels of MX, and homozygotes for CYP2D6*10 also showed an increase, although to a lesser extent. Thus, the CYP2D6*10 allele plays an important role in MX pharmacokinetics.


Similar content being viewed by others
References
Chew CY, Collett J, Singh BN (1979) Mexiletine: a review of its pharmacological properties and therapeutic efficacy in arrhythmias. Drugs 17:161–181
Jarvis B, Coukell AJ (1998) Mexiletine. A review of its therapeutic use in painful diabetic neuropathy. Drugs 56:691–707
Beckett AH, Chidomere EC (1977) The identification and analysis of mexiletine and its metabolic products in man. J Pharm Pharmacol 29:281–285
Turgeon J, Pare JR, Lalande M, Grech-Belanger O, Belanger PM (1992) Isolation and structural characterization by spectroscopic methods of two glucuronide metabolites of mexiletine after N-oxidation and deamination. Drug Metab Dispos 20:762–769
Broly F, Libersa C, Lhermitte M (1990) Mexiletine metabolism in vitro by human liver. Drug Metab Dispos 18:362–368
Broly F, Vandamme N, Libersa C, Lhermitte M (1991) The metabolism of mexiletine in relation to the debrisoquine/sparteine-type polymorphism of drug oxidation. Br J Clin Pharmacol 32:459–466
Turgeon J, Fiset C, Giguere R, Gilbert M, Moerike K, Rouleau JR, et al. (1991) Influence of debrisoquine phenotype and of quinidine on mexiletine disposition in man. J Pharmacol Exp Ther 259:789–798
Broly F, Gaedigk A, Heim M, Eichelbaum M, Morike K, Meyer UA (1991) Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol 10:545–558
Horai Y, Nakano M, Ishizaki T, Ishikawa K, Zhou H-H, Zhou B-J, et al. (1989) Metoprolol and mephenytoin oxidation polymorphisms in far eastern oriental subject: Japanese versus mainland Chinese. Clin Pharmacol Ther 46:198–207
Yokota H, Tamura S, Furuya H, Kimura S, Watanabe M, Kanazawa I, et al. (1993) Evidence for a new variant CYP2D6 allele CYP2D6 J in a Japanese population associated with lower in vivo rates of sparteine metabolism. Pharmacogenetics 3:256–263
Johansson I, Lundqvist E, Bertilsson L, Dahl ML, Sjoqvist F, Ingelman SM (1993) Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci U S A 90:11825–11829
Lai ML, Wang SL, Lai MD, Lin ET, Tse M, Huang JD (1995) Propranolol disposition in Chinese subjects of different CYP2D6 genotypes. Clin Pharmacol Ther 58:264–268
Huang JD, Chuang SK (1998) Pharmacokinetics of metoprolol in Chinese of different CYP2D6 genotypes. Clin Pharmacol Ther 63:226
Fukuda T, Yamamoto I, Nishida Y, Zhou Q, Ohno M, Takada K, et al. (1999) Effect of the CYP2D6*10 genotype on venlafaxine pharmacokinetics in healthy adult volunteers. Br J Clin Pharmacol 47:450–453
Yoon YR, Cha IJ, Shon JH, Kim KA, Cha YN, Jang IJ, et al. (2000) Relationship of paroxetine disposition to metoprolol metabolic ratio and CYP2D6*10 genotype of Korean subjects. Clin Pharmacol Ther 67:567–576
Senda C, Yamaura Y, Kobayashi K, Fujii H, Minami H, Sasaki Y, et al. (2001) Influence of the CYP2D6*10 allele on the metabolism of mexiletine by human liver microsomes. Br J Clin Pharmacol 52:100–103
Nishida Y, Fukuda T, Yamamoto I, Azuma J (2000) CYP2D6 genotypes in a Japanese population: low frequencies of CYP2D6 gene duplication but high frequency of CYP2D6*10. Pharmacogenetics 10:567–570
Steen VM, Andreassen OA, Daly AK, Tefre T, Borresen AL, Idle JR, et al. (1995) Detection of poor metabolizer-associated CYP2D6(D) gene deletion allele by long-PCR technology. Pharmacogenetics 5:215–223
Grech-Belanger O, Gilbert M, Turgeon J, LeBlanc PP (1985) Effect of cigarette smoking on mexiletine kinetics. Clin Pharmacol Ther 37:638–643
Nakajima M, Kobayashi K, Shimada N, Tokudome S, Yamamoto T, Kuroiwa Y (1998) Involvement of CYP1A2 in mexiletine metabolism. Br J Clin Pharmacol 46:55–62
Campbell NP, Kelly JG, Adgey AA, Shanks RG (1978) The clinical pharmacology of mexiletine. Br J Clin Pharmacol 6:103–108
Acknowledgements
This study was supported in part by a grant (99–2) from the Organization for Pharmaceutical Safety and Research and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otani, M., Fukuda, T., Naohara, M. et al. Impact of CYP2D6*10 on mexiletine pharmacokinetics in healthy adult volunteers. Eur J Clin Pharmacol 59, 395–399 (2003). https://doi.org/10.1007/s00228-003-0656-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0656-5