Abstract
Objective
To examine the potential effect of daidzein on CYP1A2 activity and on the pharmacokinetics of theophylline by inhibiting its metabolism.
Methods
The experiment was conducted in a single-blind, placebo-controlled, parallel study. The caffeine metabolic ratio (CMR) used as an indicator of CYP1A2 function was completed at baseline and after daidzein or placebo co-administration. A single dose of 100 mg theophylline was taken by all 20 volunteers on day 3. Thereafter, volunteers were allocated for one of two regimens. One group received 200 mg daidzein twice daily for 10 days. The other group received placebo. On day 12, the test group received 200 mg daidzein with 100 mg theophylline; the parallel group received 100 mg theophylline with placebo.
Results
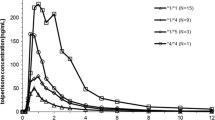
The baseline value of CMR between test group and control group did not show a difference (P=0.215). The percentage decrease in CMR ranged from −50% to 20%, with an average value of −19.8±19.7%. The percentage decrease in test group was statistically significant (P=0.009), and no significant changes were shown in the control group (t=0.12, P=0.914). By comparing the pharmacokinetic parameters of theophylline before and after daily treatment with daidzein, the effect of daidzein on the metabolism of theophylline was evident. Comparing the kinetics parameters of theophylline of day 1 (without co-medication) with those of day 12 (10-day daidzein), the AUC0–48, AUC0–∞, Cmax and t1/2 were significantly increased by 33.57±21.75% (CI, 1.21–1.46, P<0.05), 33.77±21.45% (CI, 1.20–1.46, P<0.05), 23.54±16.93% (CI, 1.23–1.52, P<0.05) and 41.39±45.92% (t=−3.19, P=0.011), respectively. The pharmacokinetic parameters of theophylline within the placebo group showed no statistically significant difference (P>0.05).
Conclusions
Daidzein, a principal isoflavone in soybean, in higher doses may inhibit CYP1A2 activity in vivo, and physicians should be aware of potential drug–food interactions.


Similar content being viewed by others
References
Adlercreutz H, Goldin BR, Gorbach SL, Hockerstedt K, Wantanabe S (1995) Soybean phytoestrogen intake and cancer risk. J Nutr 125[Suppl]:757s–770s
Adlercreutz H (1990) Western diet and western disease: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest 50:3–23
Constantinou AI, Krygier AE, Mehta RR (1998) Genistein induces maturation of cultured human breast cancer cells and prevents tumor growth in nude mice. Am J Clin Nutr 68[Suppl]:1426S–1430S
McMichichael-Phillips DF, Harding C, Morton M, Roberts SA, Howell, A, et al (1998) Effect of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr 68[Suppl]:1431S–1436S
Bigham SA, Atkinson C, Liggins J, Bluck L, Coward A (1998) Phyto-estrogens: where are we now? Br J Nutr 9:393–406
King RA, Bursill DB (1998) Plasma and urinary kinetics of the isoflavone daidzein and genistein after a single soy meal in humans. Am J Clin Nutr 67:867–872
Watanabe S, Yamaguchi M, Sobue T, Takahashi T, Miura T, Arai Y, Mazur W, Wahala K, Adrercreutz H (1998) Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). J Nutr 128:1710–1715
Adlercreutz H, van der Wildt J, Kinzel J, Attalla H, Wahala K, Makela T, Hase T, Fostsis T (1995) Lignan and isoflavonoid conjugates in human urine. J Steroid Biochem Mol Biol 54:97–103
Kulling SE, Honig DM, Simat TJ (2000) Oxidative in vitro metabolism of the soy phytoestrogens daidzein and genistein. J Agric Food Chem 48:4963–4972
Kulling SE, Honig DM, Manfred M (2001) Oxidative metabolism of the soy isoflavone daidzein and genistein in humans in vitro and in vivo. J Agric Food Chem 49:3024–3033
Pollock BG, Wylie M, Stack JA, et al (1999) Inhibition of caffeine metabolism by estrogen replacement therapy in postmenopausal women. J Clin Pharmacol 39:936–940
Patwardhan RV, Desmond PV, Wilkinson GR, Svheenker S (1980) Impaired elimination of caffeine by oral contraceptive steroids. J Lab Clin Med 95:604–608
Reitveld BC, Broekman MMM, Houben JJG, Eskes TKAB, et al (1984) Rapid onset of an increase in caffeine residence time in young woman due to oral contraceptive steroids. Eur J Clin Pharmacol 26:371–373
Fuhr U, Rost KL (1994) Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics 4:109–116
Tang-liu DD-s, Williams RL, Reigeniman S (1983) Disposition of caffeine and its metabolism in man. J Pharmacol Exp Ther 224:180–185
Steinijans VW, Hartmann M, Huber R, Radtke HWD (1991) Lack of pharmacokinetic interaction as an equivalence problem. Int J Clin Pharmacol Ther Toxicol 29:323–328
Satchell KD, Zimmer-Nedhimias L,Cai J, Heubi JE (1997) Exposure of infants to phyto-estrogens from soy-based formular. Lancet 350:23–27
Xu X, Harris KS, Wang H, Murphy PA, Hendrich S (1995) Bioavailability of soybean ispflavones depends upon gut microflora in women. J Nutr 125:2307–2315
Tjia JF, Colbert J, Back DJ (1996) Theophylline metabolism in human liver microsomes: inhibition studies. J Pharmacol Exp Ther 276:912–917
Rasmussen BB, Brosen K (1997) Theophylline has no advantages over caffeine as a putative model drug for assessing CYPIA2 activity in human. Br J Clin Pharmacol 43:253–258
Tsyrlow IB, Mikhailenko VM, Geloin HV (1994) Isozyme- and specific susceptibility of cDNA-expressed CYP1A P450s to different flavonoids. Biochim Biophys Acta 1205:325–335
Acknowledgements
Project supported by China Medical Board 99–697 and 01–755, and National Natural Science Foundation of China grant F30130210
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, WX., Li, HD. & Zhou, HH. Effect of daidzein on CYP1A2 activity and pharmacokinetics of theophylline in healthy volunteers. Eur J Clin Pharmacol 59, 237–241 (2003). https://doi.org/10.1007/s00228-003-0596-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0596-0