Abstract
Objective
To investigate the usefulness of the 3-hydroxylation of quinine as a biomarker reaction for the activity of CYP3A4 in man and to study the interindividual variation in the metabolic ratio (MR), i.e. quinine/3-hydroxyquinine.
Methods
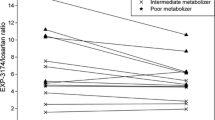
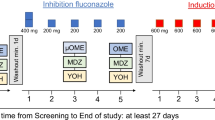
Data from a previous study (A) was used for determination of the MR of quinine in plasma and urine at different time points. In study B, 24 healthy Swedish subjects received 250 mg quinine hydrochloride first alone and later together with four other CYP probe drugs [losartan (CYP2C9), omeprazole (CYP2C19), debrisoquine (CYP2D6) and caffeine (CYP1A2)] administered on the same day. Plasma and urine samples were collected before quinine intake and 16 h thereafter and analysed for quinine and 3-hydroxyquinine using high-performance liquid chromatography. Plasma and/or urine were collected for the other probes at different time points. MRs of all the probes were determined and correlations to quinine MR were studied.
Results
In study A, the MR in plasma was stable over 96 h. The ratio increased from 5.8 to 12.2 (P=0.006) during co-administration with ketoconazole, whereas no significant difference (P=0.76) was observed during co-administration with fluvoxamine (from 5.8 to 6.0). In study B, there was no significant difference (P=0.36) between the mean MRs when quinine was given alone (4.7) or together with the four other drugs (4.5). There was a significant correlation between the MR of quinine and omeprazole sulphone formation (r=0.52, P<0.01), but not to the MRs of the other probes. There was a fivefold interindividual variability in the MR.
Conclusions
The MR of quinine in plasma or urine may serve as a stable measure of the activity of CYP3A4 in man. These results together with in vitro data show that quinine is also a specific CYP3A4 probe.




Similar content being viewed by others
References
Gellner K, Eiselt R, Hustert E, Arnold H, Koch I, Haberl M, Deglmann CJ, Burk O, Buntefuss D, Escher S, Bishop C, Koebe HG, Brinkmann U, Klenk HP, Kleine K, Meyer UA, Wojnowski L (2001) Genomic organization of the human CYP3A locus: identification of a new, inducible CYP3A gene. Pharmacogenetics 11:111–121
Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP (1994) Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270:414–423
Krishna DR, Klotz U (1994) Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet 26:144–160
Guengerich FP (1999) Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17
Bertz RJ, Granneman GR (1997) Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet 32:210–258
Streetman DS, Bertino JS Jr, Nafziger AN (2000) Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics 10:187–216
Shimada T, Oda Y, Gillam EM, Guengerich FP, Inoue K (2001) Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos 29:1176–1182
Offman EM, Freeman DJ, Dresser GK, Munoz C, Bend JR, Bailey DG (2001) Red wine–cisapride interaction: comparison with grapefruit juice. Clin Pharmacol Ther 70:17–23
Dai D, Tang J, Rose R, Hodgson E, Bienstock RJ, Mohrenweiser HW, Goldstein JA (2001) Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J Pharmacol Exp Ther 299:825–831
Sata F, Sapone A, Elizondo G, Stocker P, Miller VP, Zheng W, Raunio H, Crespi CL, Gonzalez FJ (2000) CYP3A4 allelic variants with amino acid substitutions in exons 7 and 12: evidence for an allelic variant with altered catalytic activity. Clin Pharmacol Ther 67:48–56
Eiselt R, Domanski TL, Zibat A, Mueller R, Presecan-Siedel E, Hustert E, Zanger UM, Brockmoller J, Klenk HP, Meyer UA, Khan KK, He YA, Halpert JR, Wojnowski L (2001) Identification and functional characterization of eight CYP3A4 protein variants. Pharmacogenetics 11:447–458
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E (2001) Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 27:383–391
White NJ, Chanthavanich P, Krishna S, Bunch C, Silamut K (1983) Quinine disposition kinetics. Br J Clin Pharmacol 16:399–403
Connolly PS, Shirley EA, Wasson JH, Nierenberg DW (1992) Treatment of nocturnal leg cramps. A crossover trial of quinine vs vitamin E. Arch Intern Med 152:1877–1880
Wattel E, Solary E, Hecquet B, Caillot D, Ifrah N, Brion A, Mahe B, Milpied N, Janvier M, Guerci A, Rochant H, Cordonnier C, Dreyfus F, Buzyn A, Hoang-Ngoc L, Stoppa AM, Gratecos N, Sadoun A, Stamatoulas A, Tilly H, Brice P, Maloisel F, Lioure B, Desablens B, Fenaux P et al (1998) Quinine improves the results of intensive chemotherapy in myelodysplastic syndromes expressing P-glycoprotein: results of a randomized study. Br J Haematol 102:1015–1024
Mirghani RA, Yasar U, Zheng T, Cook JM, Gustafsson LL, Tybring G, Ericsson O (2002) Enzyme kinetics for the formation of 3-hydroxyquinine and three new metabolites of quinine in vitro: 3-hydroxylation by CYP3A4 is indeed the major metabolic pathway. Drug Metab Dispos 30:1368–1371
Gascon MP, Dayer P (1991) In vitro forecasting of drugs which may interfere with the biotransformation of midazolam. Eur J Clin Pharmacol 41:573–578
Chang TK, Gonzalez FJ, Waxman DJ (1994) Evaluation of triacetyloleandomycin, alpha-naphthoflavone and diethyldithiocarbamate as selective chemical probes for inhibition of human cytochromes P450. Arch Biochem Biophys 311:437–442
Zhang H, Coville PF, Walker RJ, Miners JO, Birkett DJ, Wanwimolruk S (1997) Evidence for involvement of human CYP3A in the 3-hydroxylation of quinine. Br J Clin Pharmacol 43:245–252
Mirghani RA, Hellgren U, Westerberg PA, Ericsson O, Bertilsson L, Gustafsson LL (1999) The roles of cytochrome P450 3A4 and 1A2 in the 3-hydroxylation of quinine in vivo. Clin Pharmacol Ther 66:454–460
Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, Brown MB, Guo W, Watkins PB (1997) Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest 99:2545–2553
Ho PC, Chalcroft SC, Coville PF, Wanwimolruk S (1999) Grapefruit juice has no effect on quinine pharmacokinetics. Eur J Clin Pharmacol 55:393–398
Sowunmi A, Salako LA (1996) Effect of dose size on the pharmacokinetics of orally administered quinine. Eur J Clin Pharmacol 49:383–386
Yasar U, Forslund-Bergengren C, Tybring G, Dorado P, Llerena A, Sjoqvist F, Eliasson E, Dahl ML (2002) Pharmacokinetics of losartan and its metabolite E-3174 in relation to the CYP2C9 genotype. Clin Pharmacol Ther 71:89–98
Dalen P, Dahl ML, Eichelbaum M, Bertilsson L, Wilkinson GR (1999) Disposition of debrisoquine in Caucasians with different CYP2D6-genotypes including those with multiple genes. Pharmacogenetics 9:697–706
Fuhr U, Rost K (1994) Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics 4:109–116
Chang M, Dahl ML, Tybring G, Gotharson E, Bertilsson L (1995) Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics 5:358–363
Mirghani RA, Ericsson O, Cook J, Yu P, Gustafsson LL (2001) Simultaneous determination of quinine and four metabolites in plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 754:57–64
Scordo MG, Spina E, Romeo P, Dahl ML, Bertilsson L, Johansson I, Sjoqvist F (2000) CYP2D6 genotype and antipsychotic-induced extrapyramidal side effects in schizophrenic patients. Eur J Clin Pharmacol 56:679–683
Dresser GK, Spence JD, Bailey DG (2000) Pharmacokinetic–pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 38:41–57
Wrighton SA, Ring BJ (1994) Inhibition of human CYP3A catalyzed 1'-hydroxy midazolam formation by ketoconazole, nifedipine, erythromycin, cimetidine, and nizatidine. Pharm Res 11:921–924
Watkins PB, Murray SA, Winkelman LG, Heuman DM, Wrighton SA, Guzelian PS (1989) Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. Studies in rats and patients. J Clin Invest 83:688–697
Maenpaa J, Hall SD, Ring BJ, Strom SC, Wrighton SA (1998) Human cytochrome P450 3A (CYP3A) mediated midazolam metabolism: the effect of assay conditions and regioselective stimulation by alpha- naphthoflavone, terfenadine and testosterone. Pharmacogenetics 8:137–155
Paine MF, Davis CL, Shen DD, Marsh CL, Raisys VA, Thummel KE (2000) Can oral midazolam predict oral cyclosporine disposition? Eur J Pharm Sci 12:51–62
White N (1992) Antimalarial pharmacokinetics and treatment regimens. Br J Clin Pharmacol 34:1–10
Damkier P, Brosen K (2000) Quinidine as a probe for CYP3A4 activity: intrasubject variability and lack of correlation with probe-based assays for CYP1A2, CYP2C9, CYP2C19, and CYP2D6. Clin Pharmacol Ther 68:199–209
Branch RA, Adedoyin A, Frye RF, Wilson JW, Romkes M (2000) In vivo modulation of CYP enzymes by quinidine and rifampin. Clin Pharmacol Ther 68:401–411
Otton SV, Inaba T, Kalow W (1984) Competitive inhibition of sparteine oxidation in human liver by beta-adrenoceptor antagonists and other cardiovascular drugs. Life Sci 34:73–80
Spina E, Steiner E, Dumont E, Dahlqvist R (1989) Inhibition of desipramine 2-hydroxylation by quinidine and quinine in rapid and slow debrisoquine hydroxylators. Psychopharmacol Ser 7:201–205
Zhao XJ, Yokoyama H, Chiba K, Wanwimolruk S, Ishizaki T (1996) Identification of human cytochrome P450 isoforms involved in the 3-hydroxylation of quinine by human liver microsomes and nine recombinant human cytochromes P450. J Pharmacol Exp Ther 279:1327–1334
Zhao XJ, Ishizaki T (1997) Metabolic interactions of selected antimalarial and non-antimalarial drugs with the major pathway (3-hydroxylation) of quinine in human liver microsomes. Br J Clin Pharmacol 44:505–511
Mirghani RA, Yasar U, Zheng T, Cook JM, Gustafsson LL, Tybring G, Ericsson O (2002) Enzyme kinetics for the formation of 3-hydroxyquinine and three new metabolities of quinine in vitro; 3-hydroxylation by CYP3A4 is indeed the major metabolic pathway. Drug Metab Dispos 30(12):1368–1371
Brosen K, Skjelbo E, Rasmussen BB, Poulsen HE, Loft S (1993) Fluvoxamine is a potent inhibitor of cytochrome P4501A2. Biochem Pharmacol 45:1211–1214
Paintaud G, Alvan G, Ericsson O (1993) The reproducibility of quinine bioavailability. Br J Clin Pharmacol 35:305–307
Bertilsson L, Tybring G, Widen J, Chang M, Tomson T (1997) Carbamazepine treatment induces the CYP3A4 catalysed sulphoxidation of omeprazole, but has no or less effect on hydroxylation via CYP2C19. Br J Clin Pharmacol 44:186–189
Bottiger Y, Tybring G, Gotharson E, Bertilsson L (1997) Inhibition of the sulfoxidation of omeprazole by ketoconazole in poor and extensive metabolizers of S-mephenytoin. Clin Pharmacol Ther 62:384–391
Kinirons MT, O'Shea D, Downing TE, Fitzwilliam AT, Joellenbeck L, Groopman JD, Wilkinson GR, Wood AJ (1993) Absence of correlations among three putative in vivo probes of human cytochrome P4503A activity in young healthy men. Clin Pharmacol Ther 54:621–629
Stein CM, Kinirons MT, Pincus T, Wilkinson GR, Wood AJ (1996) Comparison of the dapsone recovery ratio and the erythromycin breath test as in vivo probes of CYP3A activity in patients with rheumatoid arthritis receiving cyclosporine. Clin Pharmacol Ther 59:47–51
Hunt CM, Watkins PB, Saenger P, Stave GM, Barlascini N, Watlington CO, Wright JT Jr, Guzelian PS (1992) Heterogeneity of CYP3A isoforms metabolizing erythromycin and cortisol. Clin Pharmacol Ther 51:18–23
Westlind A, Lofberg L, Tindberg N, Andersson TB, Ingelman-Sundberg M (1999) Interindividual differences in hepatic expression of CYP3A4: relationship to genetic polymorphism in the 5'-upstream regulatory region. Biochem Biophys Res Commun 259:201–205
Acknowledgements
The study was financially supported by the Swedish Agency for Research Cooperation with Developing Countries Grant Bil Tz 16/98 75007059, SWE 1997–221, 1998–394, 1999–260, 2000–175, the Swedish Medical Research Council grant no. 3902, Karolinska Institutet, the National Institutes of Health, USA (1R01 GM60548–02), and Pfizer Ltd, Sandwich, UK. Our thanks are extended to the research nurses Anneli Wahlberg and Katarina Andersson for their excellent help in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirghani, R.A., Ericsson, Ö., Tybring, G. et al. Quinine 3-hydroxylation as a biomarker reaction for the activity of CYP3A4 in man. Eur J Clin Pharmacol 59, 23–28 (2003). https://doi.org/10.1007/s00228-003-0575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0575-5