Abstract
Objective
To describe the methodological problems in collecting retrospectively comparable data on drug use and to compare the use of antibacterials in some European countries.
Methods
A spreadsheet was distributed in 2000 through the European Drug Utilisation Research Group (EuroDURG) network, requesting 1994–1999 data on use of antibacterials for systemic use (ATC group J01), from ambulatory, hospital, or total care, aggregated at ATC 4th level, and presented in defined daily doses per 1000 inhabitants per day (in the 1999 ATC/DDD version or specified other version).
Results
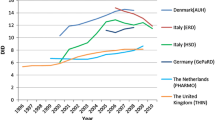
The network was able to provide national utilization data for two or more years in the requested period from 16 countries (4 only from primary care, 3 both from primary care and total use, and 9 only total use data). The main methodological problems identified were: use of divergent ATC/DDD versions, divergent assignment of DDDs for combination products and the use of unofficial or national DDDs. It was possible to correct for the different ATC/DDD versions to some extent, except for the cephalosporin group (not included in the analysis), as the collection of data at the ATC 4th level precluded recalculation of DDDs. In the seven countries with primary care data the total J01 antibacterials use varied by a factor of 2.5 (Belgium 23.4 and The Netherlands 9.5 DDDs per 1000inhabitants per day). The use of J01A tetracyclines varied fourfold, and the use of J01C penicillins and J01F macrolides and lincosamides approximately threefold. Significant reduction over time was seen in J01A and an increase in J01F.
Conclusions
In the scientific and regulatory community it is still difficult to perform a valid and comprehensive cross-national collection of utilization data on antibacterials. White spots on the European map persist for ambulatory care data, and data are missing for the hospital sector in most countries. For a thorough explanation of the considerable intercountry variability (especially in antibacterial subgroups and time trends analysis) a sustained and concerted effort is necessary to implement a validation process of the ATC/DDD use in the various countries and to adopt a common methodological approach to the collection of utilization data at the substance level (ATC 5th level).




Similar content being viewed by others
References
Cars O, Mölstad S, Melander A (2001) Variation in antibiotic use in the European Union. Lancet 357:1851–1853
WHO Collaborating Centre for Drug Statistics Methodology (Norway) (2002) Guidelines for ATC classification and DDD assignment. WHO Collaborating Centre, Oslo
WHO Collaborating Centre for Drug Statistics Methodology (Norway) (2002) ATC index with DDDs. WHO Collaborating Centre, Oslo
WHO Collaborating Centre for Drug Statistics Methodology (Norway) (1999) ATC index with DDDs. WHO Collaborating Centre, Oslo
Nordic Medico Statistical Committee (Denmark) (2001) Health Statistics in the Nordic Countries. Report 61, 2001. NOMESCO, Copenhagen
Mölstad S, Lundborg CS, Karlsson AK, Cars O (2002) Antibiotic prescription rates vary markedly between 13 European countries. Scand J Infect Dis 34:366–371
Rønning M, Salvesen Blix H, Harbø BT, Strøm H (2000) Different versions of the anatomical therapeutic chemical classification system and the defined daily dose—are drug utilisation data comparable? Eur J Clin Pharmacol 56:723–727
Folino-Gallo P, Walley T, Frolich JC, Carvajal A, Edwards IR (2001) Availability of medicines in the European Union: results from the EURO-Medicines project. Eur J Clin Pharmacol 57:441–446
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rønning, M., Blix, H.S., Strøm, H. et al. Problems in collecting comparable national drug use data in Europe: the example of antibacterials. Eur J Clin Pharmacol 58, 843–849 (2003). https://doi.org/10.1007/s00228-003-0572-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0572-8