Abstract
Aim
The aim of the current retrospective study was to assess the influence of polymorphic drug metabolism as assessed by genotyping, on the on the utilisation of psychotropic drugs in hospitalised psychiatric patients. The utilisation of psychotropic drugs was assessed using pharmacy records with emphasis on the number of prescriptions and prescriptions for possible side effects.
Methods
CYP2D6 genotype was assessed in 241 psychiatric patients by investigation for the five most common allelic variants (CYP2D6*3, *4, *6, *7, *8) and the presence of gene duplication using allele-specific polymerase chain reaction. Data concerning the pharmacotherapy of the patients were retrieved from the pharmacy information system. Data was analysed on differences observed in pharmacy records concerning the different metabolic classes: ultra rapid metabolisers (UMs), extensive metabolisers (EMs) and poor metabolisers (PMs).
Results
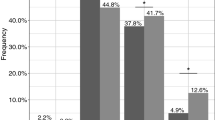
For CYP2D6, 2.5% was UM (95% CI: 0.5–4.5%, n=6) and 8.3% was PM (95% CI: 4.8–11.8%, n=20). Drugs metabolised by CYP2D6 were less frequently prescribed in PMs than EMs (21.1% vs 33.6%, P=0.023). The average duration of prescriptions was significantly lower in PMs than EMs (54 days vs 106 days, P=0.010). Between UMs and EMs, no significant differences were found, although a similar tendency was observed. With regard to dose, no consistent differences were observed between the CYP2D6 genotype classes. Drugs against Parkinsonian-like side effects were given twice as frequently in PMs as EMs (6.9% vs 3.4%, P=0.045).
Conclusions
Patients with impaired CYP2D6 metabolism received fewer CYP2D6 drugs. PMs were more prone to Parkinsonian-like side effects as evidenced by more prescriptions for drugs combating these side effects. Dose titrations were not often used to compensate for genetic polymorphisms. Pharmacy records might be a useful tool to detect differences related to polymorphic metabolism.


Similar content being viewed by others
References
Bertz RJ, Granneman GR (1997) Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet 32:210–258
Daly AK, Cholerton S, Gregory W, Idle JR (1993) Metabolic polymorphisms. Pharmacol Ther 57:129–160
Meyer UA, Zanger UM (1997) Molecular mechanism of genetic polymorphism of drug metabolism. Annu Rev Pharmacol Toxicol 37:269–296
Daly AK, Brockmöller J, Broly F, Eichelbaum M, Evans WE, Gonzalez FJ, Huang J-D, Idle JR, Ingelman-Sundberg M, Ishizaki T, Jacqz-Aigrain E, Meyer UA, Nebert DW, Steen VM, Wolf CR, Zanger UM (1996) Nomenclature for human CYP2D6 alleles. Pharmacogenetics 6:193–201
Griese E-U, Zanger UM, Brudermanns U, Gaedigk A, Mikus G, Mörike K, Stüven T, Eichelbaum M (1998) Assessment of the predictive power of genotypes for the in-vivo catalytic function of CYP2D6 in German population. Pharmacogenetics 8:15–26
Edeki T (1996) Clinical importance of genetic polymorphism of drug oxidation. Mount Sinai J Med 63:290–300
Spina E, Caputi AP (1994) Pharmacogenetic aspects in the metabolism of psychotropic drug: pharmacokinetics and clinical implications. Pharmacol Res 29:121–137
Meyer UA, Amrein R, Balant LP, Bertilsson L, Eichelbaum M, Guenter TW, Henauer S, Jackson P, Laux G, Mikkelsen H, Peck C, Pollock B, Priest R, Sjöqvist F, Delini-Stula A (1996) Antidepressant and drug-metabolizing enzymes—expert group report. Acta Psychiatr Scand 93:71–79
Spina E, Gitto C, Avenoso A, Campo GM, Caputi AP, Perucca E (1997) Relationship between plasma desipramine levels, CYP2D6 phenotype and clinical response to desipramine: a prospective study. Eur J Clin Pharmacol 51:395–398
Cohen LJ, DeVane CL (1996) Clinical implications of antidepressant pharmacokinetics and pharmacogenetics Ann Pharmacother 30:1471–1480
Dahl ML, Bertilsson L (1993) Genetically variable metabolism of antidepressants and neuroleptic drug in man. Pharmacogenetics 3:61–70
Dahl-Puustinen ML, Lidén A, Alm C, Nordin C, Bertilsson L (1989) Disposition of perphenazine is related to polymorphic debrisoquine hydroxylation in human beings. Clin Pharmacol Ther 46:78–81
Bertilsson L, Dahl ML (1996) Polymorphic drug oxidation: relevance to the treatment of psychiatric disorders. CNS Drugs 5:200–223
Vandel P, Haffen E, Vandel S, Bonin B, Nezelhof S, Sechter D, Broly F, Biouard P, Dalery J (1999) Drug extrapyrimidal side effects. CYP2D6 genotypes and phenotypes. Eur J Clin Pharmacol 55:659–665
Chen S, Chou WH, Blouin RA, Mao Z, Humpries LL, Meek QC, Neill JR, Martin WL, Hays LR, Wedlund PJ (1996) The cytochrome P450 2D6 (CYP2D6) enzyme polymorphism: screening costs and influence on clinical outcomes in psychiatry. Clin Pharmacol Ther 60:522–534
De Leon J, Barnhill J, Rogers T, Boyle J, Chou WH, Wedlund PJ (1998) Pilot study of cytochrome P450–2D6 genotype in a psychiatric state hospital. Am J Psychiatry 155:1278–1280
Chou WH, Yan FX, De Leon J, Barnhill J, Roger T, Cronin M, Pho M, Xiao V, Ryder TB, Liu WW, Teiling C, Wedlund PJ (2000) Extension of a pilot study: impact from the cytochrome P450 2D6 polymorphism on outcome and costs associated with severe mental illness. J Clin Psychopharmacol 20:246–251
Lau HS, de Boer A, Beuning KS, Porsius A (1997) Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol 50:619–625
Stüven T, Griese E-U, Kroemer HK, Eichelbaum M, Zanger UM (1996) Rapid detection of CYP2D6 null alleles by long distance and multiplex-polymerase chain reaction. Pharmacogenetics 6:417–421
Løvlie R, Daly AK, Molven A, Idle JR, Steen VM (1996) Ultrarapid metabolizers of debrisoquin: characterisation and PCR-based detection of alleles with duplication of the CYP2D6 gene. FEBS Lett 392:30–34
Steijns LWS, van der Weide J (1998) Ultrarapid drug metabolism: PCR-based detection of CYP2D6 gene duplication. Clin Chem 44:914–917
Flockhart DA Web site of the Georgetown University Medical Center (Washington DC, USA) on http://www.dml.georgetown.edu/depts/pharmacology/davetab.html
WHO (1997) WHO collaborating Centre for Drug Statistics Methodology. ATC index with DDD's (Oslo, Norway)
Tamminga WJ, Wemer J, Oosterhuis B, Wieling J, Wilffert B, De Leij LFMH, De Zeeuw RA, Jonkman JHG (1999) CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol 55:177–184
Arthur H, Dahl ML, Siwers B, Sjöqvist F (1995) Polymorphic drug metabolism in schizophrenic patients with tardive dyskinesia. J Clin Pschopharmacol 15:211–216
Spiget O, Hedenmalm K, Dahl ML, Wiholm BE, Dahlqvist R (1997) Seizures and myoclonus associated with antidepressant treatment: assessment of potential risk factors, including CYP2D6 and CYP2C19 polymorphisms, and treatment with CYP2D6 inhibitors. Acta Psychiatr Scand 96:379–384
Gex-Fabry M, Balant-Gorgia AE, Balant LP (1999) Clomipramine concentrations as a predictor of delayed response: a naturalistic study. Eur J Clin Pharmacol 54:895–902
Aitchison JA, Munro J, Wright P, Smith S, Makoff AJ, Sachse C, Sham PC, Murray RM, Collier DA, Kerwin RW (1999) Failure to respond to treatment with typical antipsychotics is not associated with CYP2D6 ultrarapid hydroxylation. Br J Clin Pharmacol 48:388–394
Lessard E, Yessine MA, Hamelin BA, O'Hara G, LeBlanc J, Turgeon J (1999) Influence of CYP2D6 activity on the disposition and cardiovascular toxicity of the antidepressant agent venlafaxine in humans. Pharmacogenetics 9:435–443
Sekine Y, Rikihisa T, Ogata H, Echizen H, Arakawa Y (1999) Correlations between in vitro affinity of antipsychotics to various central neurotransmitter receptors and clinical incidence of their adverse drug reactions. Eur J Clin Pharmacol 55:583–587
Arranz MJ, Munro J, Birkett J, Bolonna A, Mancama D, Sodhi M, Lesch KP, Meyer JFW, Sham P, Collier DA, Muray RM, Kerwin RW (2000) Pharmacogenetic prediction of clozapine response. The Lancet 355:1615–1616
Dahl M-L, Sjöqvist F (2000) Pharmacogenetic methods as a complement to therapeutic monitoring of antidepressants and neuroleptics. Ther Drug Monit 22:114–117
Kirchheiner J, Brøsen K, Dahl ML, Gram LF, Kasper S, Roots I, Sjöqvist F, Spina E, Bröckmöller J (2001) CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 104:173–192
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamminga, W.J., Wemer, J., Oosterhuis, B. et al. Polymorphic drug metabolism (CYP2D6) and utilisation of psychotropic drugs in hospitalised psychiatric patients: a retrospective study. Eur J Clin Pharmacol 59, 57–64 (2003). https://doi.org/10.1007/s00228-003-0562-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0562-x