Abstract
Purpose
The antiepileptic drugs (AEDs) retigabine (RGB) and lamotrigine (LTG) undergo predominantly N-glucuronidation and renal excretion. This study was performed to evaluate potential pharmacokinetic interactions between both AEDs.
Methods
Twenty-nine healthy male subjects participated in the study. Group A (n=14) received single oral 200-mg RGB doses on day 1 and day 7, and 25 mg o.i.d. LTG on days 3–8. Group B (n=15) received single oral 200-mg LTG doses on day 1 and day 17, and was up-titrated to 300 mg RGB b.i.d. on days 6–20. Blood samples were collected to compare the pharmacokinetics of both AEDs and the N-acetyl metabolite of RGB (AWD21–360) after single and concomitant treatments.
Results
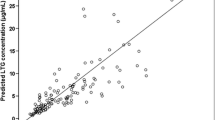
RGB was rapidly absorbed and eliminated with a mean half-life (t1/2) of 6.3±1.1 h and an apparent clearance (CL/F) of 0.69±1.4 l/h/kg. Under co-administration of LTG, mean RGB t1/2 and area under the plasma concentration–time curve (AUC) were increased by 7.5% (P=0.045) and 15% (P=0.006), respectively, while CL/F was decreased by 13% (P=0.06). Consistent results were obtained for AWD21–360. LTG was moderately rapidly absorbed, eliminated with a mean t1/2 of 37±10.4 h and a CL/F of 0.028±0.007 l/h/kg. Under co-administration of RGB, mean LTG t1/2 and AUC decreased by 15% and 18%, respectively, while CL/F increased by 22% (all parameters, P=0.001).
Conclusions
RGB and LTG exhibit a modest pharmacokinetic interaction on each other. The slight decline in RGB clearance due to LTG is believed to result from competition for renal elimination rather than competition for glucuronidation. The induction of LTG clearance due to retigabine was unexpected since RGB did not show enzyme induction in various other drug–drug interaction studies. Further studies in patients are needed to assess the clinical relevance of these findings for concomitant treatment with both drugs in the upper recommended dose range.





Similar content being viewed by others
References
Rundfeldt C, Netzer R (2000) The novel anticonvulsant retigabine activates M-currents in Chinese hamster ovary cells transfected with human KCNQ2/3 subunits. Neurosci Lett 281:73–76
Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen US, Dixon JE, Mc Kinnon D (1998) KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282:1890–1893
Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA et al (2000) Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol 58:253–262
Wickenden AD, Yu WF, Zou A, Jegla T, Wagoner PK et al (2000) Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol 58:591–600
Wickenden AD, Zou AR, Wagoner PK, Jegla T (2001) Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br J Pharmacol 132:381–384
Hirsch E, De Saint-Martin A, Marescaux C (1999) Benign neonatal familial convulsions: a model of idiopathic epilepsy. Rev Neurol (Paris) 155:463–467
Rundfeldt C, Netzer R (2000) Investigation into the mechanism of action of the new anticonvulsant retigabine: interaction with GABAergic and glutamatergic neuro-transmission and with voltage gated Na+ and Ca++ channels. Arzneimittelforschung 50:1063–1070
Kapetanovic IM, Rundfeldt C (1996) A new anticonvulsant compound. CNS Drug Rev 2:308–321
Hermann R, Schneider E, Menth M, Knebel N, Ferron GM, Borlak J et al (2002) Retigabine. In: Bialer M et al (eds) Progress report on new antiepileptic drugs: a summary of the Sixth Eilat Conference (EILAT VI). Epilepsy Res 51:36–38
McNeilly PJ, Torchin CD, Anderson LW, Kapetanovic IM, Kupferberg HJ, Strong JM (1997) In vitro glucuronidation of D-23129, a new anticonvulsant, by human liver microsomes and liver slices. Xenobiotica 27:431–441
Hiller A, Nguyen N, Strassburg CP, Li Q, Jainta H, Pechstein B et al (1999) Retigabine N-glucuronidation and its potential role in enterohepatic circulation. Drug Metab Dispos 27:605–612
Ferron GM, Paul J, Fruncillo R, Richards L, Knebel NG, Getsy J et al (2002) Multiple-dose linear dose-proportional pharmacokinetics of retigabine in healthy volunteers. J Clin Pharm 42:175–182
Magdalou J, Herber R, Bidault R, Siest G (1992) In vitro N-glucuronidation of a novel antiepileptic drug, lamotrigine, by human liver microsomes. J Pharmacol Exp Ther 260:1166–1173
Green MD, Tephly TR (1998) Glucuronidation of amine substrates by purified and expressed UDP–glucuronyltransferase proteins. Drug Metab Dispos 26:860–867
Peck AW (1991) Clinical pharmacology of lamotrigine. Epilepsia 32:S9–S12
Elwes RD, Binnie CD (1996) Clinical pharmacokinetics of newer antiepileptic drugs: lamotrigine, vigabatrin, gabapentin, and oxcarbazepine. Clin Pharmacokinet 30:403–415
Rambeck B, Wolf P (1993) Lamotrigine clinical pharmacokinetics. Clin Pharmacokinet 25:433–443
Richens A (1992) Pharmacokinetics of lamotrigine. In: Richens A (ed) Clinical update on lamotrigine: a novel entiepileptic agent. Wells Medical Ltd, Tunbridge Wells, pp 21–27
Yau MK, Adams MA, Wargin WA, Lai AA (1992) A single-dose and steady-state pharmacokinetic study of lamictal in healthy male volunteers. Third International Cleveland Clinic, Bethel Epilepsy Symposium, Cleveland, OH, USA
Knebel NG, Grieb S, Leisenheimer S, Locher M (2000) Determination of retigabine and its acetyl metabolite in biological matrices by on-line solid-phase extraction (column switching) liquid chromatography with tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 748:97–111
Sallustio BC, Morris RG (1997) High performance liquid chromatography quantitation of plasma lamotrigine concentrations: application measuring trough concentrations in patients with epilepsy. Ther Drug Monit 19:688–693
Lensmeyer GL, Gidal BE, Weibe DA (1997) Optimized high performance liquid chromatography method for determination of lamotrigine in serum with concomitant determination of phenytoin, carbamazepine and carbamazepine-epoxide. Ther Drug Monit 19:292–300
Rowland M, Tozer TN (1989) Clinical pharmacokinetics: concepts and applications, 2nd edn. Lea & Febiger, Philadelphia
Schuirmann D (1987) A comparison of the two one-sided test procedures and the power approach for assessing the equivalence of average bioavailability. J Pharm Biopharm 15:657–680
Committee for proprietary medicinal products (1995) Note for guidance on the investigation of drug interactions. CPMP/EWP/560/95
Hussein Z, Posner J (1997) Population pharmacokinetics of lamotrigine monotherapy in patients with epilepsy: retrospective analysis of routine monitoring data. Br J Clin Pharmacol 43:457–465
Ferron GM, Paul J, Richards L, Getsy J, Troy S (2001) Retigabine does not alter the pharmacokinetics of a low dose oral contraceptive in women. Neurology 56[Suppl 3]:P05.052
Ferron GM, Patat A, Parks V, Troy S (2001) Lack of pharmacokinetic interaction between retigabine and phenobarbital at steady-state in healthy subjects. Clin Pharmacol Ther 69:48
Hermann R, Ferron GM, Erb K, Knebel N, Ruus P, Paul J, Richards L, Cnota HP, Troy S (2003) Effects of age and sex on the disposition of retigabine. Clin Pharmacol Ther 73:61–70
Potschka H, Fedrowitz M, Loscher W (2002) P-glycoprotein mediated efflux of phenobarbital, lamotrigine, and felbamate at the blood–brain barrier: evidence from microdialysis experiments in rats. Neurosci Lett 327:173–176
Acknowledgements
The study was sponsored by ASTA Medica AG (predecessor of VIATRIS GmbH & Co. KG), Frankfurt am Main, Germany, and Wyeth Research, Philadelphia, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermann, R., Knebel, N.G., Niebch, G. et al. Pharmacokinetic interaction between retigabine and lamotrigine in healthy subjects. Eur J Clin Pharmacol 58, 795–802 (2003). https://doi.org/10.1007/s00228-003-0558-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0558-6