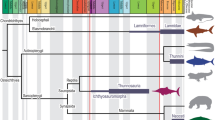
Abstract
Macroecological studies have primarily focused on investigating the relationships between body size and geographic distribution on large scales, including regional, continental, and even global levels. While the majority of these studies have been conducted on terrestrial species, a limited number of studies have been carried out on aquatic species, and even fewer have considered the importance of phylogeny in the observed patterns. Cephalopods provide a good model for examining these macroecological patterns due to their large geographic and bathymetric ranges, wide range of body sizes, as well as diverse fin sizes and shapes. In this study, we assess the relationships between mantle length, fin size, and hatchling size with the geographic and bathymetric distribution of 30 squid species from the worldwide distributed family Loliginidae. To test a macroecological hypothesis, we evaluated the phylogenetic signal and correlated evolution to assess the role of biological traits in squid distribution, using a molecular phylogeny based on two mitochondrial and one nuclear genes. Biological traits (mantle length and fin size) exhibit high phylogenetic signals, while distribution demonstrates low signal. The correlation analyses revealed the existence of a relationship between adult mantle length and fin size with geographic and bathymetric distribution, but not with hatchling size. The geographic distribution of loliginid squids evolved in relation to mantle length, where larger squids with large fins (e.g. Sepioteuthis) have wide distributions, while small-finned species (e.g. Pickfordiateuthis) have narrow distributions. This study paves the way for exploring similar relationships in other squid families or other marine swimming animals.






Similar content being viewed by others
Data availability
All data of this article is in supplementary files.
References
Anderson FE (2000a) Phylogenetic relationships among loliginid squids (Cephalopoda: Myopsida) based on analyses of multiple data sets. Zool J Linnean Soc 130:603–633
Anderson FE (2000b) Phylogeny and historical biogeography of the loliginid squids (Mollusca: Cephalopoda) based on mitochondrial DNA sequence data. Mol Phylogenet Evol 15(2):191–214
Anderson EJ, Demont ME (2000) The mechanics of locomotion in the squid Loligo pealei: locomotory function and unsteady hydrodynamics of the jet and intra mantle pressure. J Exp Biol 203(18):2851–2863
Anderson FE, Marian JEA (2020) The grass squid Pickfordiateuthis pulchella is a paedomorphic loliginid. Mol Phylogenet Evol 147:106801
Aoki M, Imai H, Naruse T, Ikeda Y (2008) Low genetic diversity of oval squid, Sepioteuthis cf. lessoniana (Cephalopoda: Loliginidae), in Japanese waters inferred from a mitochondrial DNA non-coding region. Pac Sci 62:403–411
Bartol IK, Patterson MR, Mann R (2001) Swimming mechanics and behavior of the shallow-water brief squid Lolliguncula brevis. J Exp Biol 204(21):3655–3682
Böhning-Gaese K, Caprano T, Van Ewijk K, Veith M (2006) Range size: disentangling current traits and phylogenetic and biogeographic factors. Am Nat 167:555–567
Boyle P, Rodhouse P (2005) Cephalopods. Ecology and fisheries. Blackwell Publishing, Oxford
Brakoniecki TF (1996) A revision of the genus Pickfordiateuthis Voss, 1953 (Cephalopoda; Myopsida). Bull Mar Sci 58(1):9–28
Brakoniecki TF (1986) A generic revision of the family Loliginidae (Cephalopoda: Myopsida) based primarily on the comparative morphology of the hectocotylus. Ph.D. Dissertation, University of Miami, Coral Gables, FL. p 163.
Brierley AS, Thorpe JP, Pierce GJ, Clarke MR, Boyle PR (1995) Genetic variation in the neritic squid Loligo forbesi (Myopsida: Loliginidae) in the northeast Atlantic Ocean. Mar Biol 122:79–86
Brown JH (1995) Macroecology. University of Chicago Press, Chicago
Brown JH, Stevens GC, Kaufman DM (1996) The geographic range: size, shape, boundaries, and internal structure. Annu Rev Ecol Evol Syst 27:597–423
Carrasco SA, Bravo M, Ibáñez CM, Zapata-Hernández G (2021) Discrete spawning aggregations of the loliginid squid Doryteuthis gahi reveal life-history interactions of a dwarf morphotype at the center of its distribution range. Front Mar Sci 7:1251
Cheng SH, Anderson FE, Bergman A, Mahardika GN, Muchlisin ZA, Dang BT, Calumpong HP, Mohamed KS, Sasikumar G, Venkatesan V, Barber PH (2014) Molecular evidence for co-occurring cryptic lineages within the Sepioteuthis cf. lessoniana species complex in the Indian and Indo-West Pacific Oceans. Hydrobiologia. 725(1):165–188
Clarke MR (1988) Evolution of buoyancy and locomotion in recent cephalopods. paleontology and neontology of Cephalopoda. The Mollusca 12:203–213
Cones SF, Zhang D, Shorter KA, Katija K, Mann DA, Jensen FH, Fontes J, Afonso P, Mooney TA (2022) Swimming behaviors during diel vertical migration in veined squid Loligo forbesii. Mar Ecol Prog Ser 691:83–96
Costa TA, Sales JB, Markaida U, Granados-Amores J, Gales SM, Sampaio I, Vallinoto M, Rodrigues-Filho LFS, Ready JS (2021) Revisiting the phylogeny of the genus Lolliguncula Steenstrup 1881 improves understanding of their biogeography and proves the validity of Lolliguncula argus Brakoniecki & Roper, 1985. Mol Phylogenet Evol 154:106968
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Annu Rev Mar Sci 1(1):443–466
Daniel TL (1988) Forward flapping flight from flexible fins. Can J Zool 66(3):630–638
DeMont ME, Hokkanen JEI (1992) Hydrodynamics of animal movement. In: Biewener AA (ed) Biomechanics (structures and systems): a practical approach. Oxford University Press, New York, pp 263–284
Diniz-Filho JAF, Tôrres NM (2002) Phylogenetic comparative methods and the geographic range size–body size relationship in New World terrestrial Carnivora. Evol Eco. 16:351–367
Flaspohler GE, Caruso F, Mooney TA, Katija K, Fontes J, Afonso P, Shorter KA (2019) Quantifying the swimming gaits of veined squid (Loligo forbesii) using bio-logging tags. J Exp Biol 222(24):jeb198226
Floeter SR, Rocha LA, Robertson DR, Joyeux JC, Smith-Vaniz WF, Wirtz P, Edwards AJ, Barreiros JP, Ferreira CEL, Gasparini JL, Brito A, Falcón JM, Bowen BW, Bernardi G (2008) Atlantic reef fish biogeography and evolution. J Biogeogr 35:22–47
Foyle TP, ’O’Dor RK, (1988) Predatory strategies of squid (Illex illecebrosus) attacking small and large fish. Mar Freshw Behav Physiol 13(2):155–168
Garcia-Mayoral E, Roura Á, Ramilo A, Gonzalez AF (2020) Spatial distribution and genetic structure of loliginid paralarvae along the Galician coast (NW Spain). Fish Res 222:105406
Gaston KJ (2003) The structure and dynamics of geographic ranges. Oxford University Press
Grafen A (1989) The phylogenetic regression. Philos Trans R Soc Lond B Biol Sci 326:119–157. https://doi.org/10.1098/rstb.1989.0106
Hanlon RT (1998) Mating systems and sexual selection in the squid Loligo: how might commercial fishing on spawning squids affect them? Calif Coop Ocean Fish Investig Rep 39:92–100
Hanlon RT, Messenger JB (1996) Cephalopod Behaviour. Cambridge University Press, New York
Hansen TA (1980) Influence of larval dispersal and geographic distribution on species longevity in neogastropods. Paleobiology 6(2):193–207
Harrop J, Vecchione M, Felley JD (2014) In situ observations on behaviour of the ommastrephid squid genus Illex (Cephalopoda: Ommastrephidae) in the northwestern Atlantic. J Nat Hist 48(41–42):2501–2516. https://doi.org/10.1080/00222933.2014.937367
Herke SW, Foltz DW (2002) Phylogeography of two squid (Loligo pealei and L. plei) in the Gulf of Mexico and northwestern Atlantic Ocean. Mar Biol 140:103–115
Hernández CE, Rodríguez-Serrano E, Avaria-Llautureo J, Inostroza-Michael O, Morales-Pallero B, Boric-Bargetto D et al (2013) Using phylogenetic information and the comparative method to evaluate hypotheses in macroecology. Methods Ecol Evol 4:401–415
Hoar JA, Sim E, Webber DM, O’Dor RK (1991) The role of fins in the competition between squid and fish. In: Maddock L, Bone Q, Rayner JMV (ed) Mechanics and physiology of animal swimming. Pp 27–43. ISSN/ISBN: 0-521-46078-6.
Hoorn C, Wesselingh FP, Hovikoski J, Guerrero J (2010) The development of the iogeogra mega-wetland (Miocene; Brazil, Colombia, Peru, Bolivia). In: Hoorn C, Wesselingh FP (eds) Amazonia, landscape and species evolution: a look into the past. Wiley, New York, pp 123–142. https://doi.org/10.1002/9781444306408.ch8
Ibáñez CM, Camus PA, Rocha R (2009) Diversity and distribution of cephalopod species of the coast off Chile. Mar Biol Res 5:374–384
Ibáñez CM, Argüelles J, Yamashiro C, Adasme L, Céspedes R, Poulin E (2012) Spatial genetic structure and demographic inference of the Patagonian squid Doryteuthis gahi in the Southeastern Pacific Ocean. J Mar Biol Ass UK 92:197–203
Ibáñez CM, Rezende E, Sepúlveda RD, Avaria-Llautureo J, Hernández CE, Sellanes J, Poulin E, Pardo- Gandarillas MC (2018) Thorson’s rule, life history evolution and diversification of benthic octopuses (Cephalopoda: Octopodoidea). Evolution 72–9:1829–1839
Ibáñez CM, Braid H, Carrasco SA, López-Córdova D, Torretti G, Camus PA (2019) Zoogeographic patterns of pelagic oceanic cephalopods along the eastern Pacific Ocean. J Biogeogr 46:1260–1273
Ibáñez CM, Díaz-Santana-Iturrios M, Carrasco SA, Fernández-Álvarez FA, López-Córdova DA, Cornejo CF, Ortiz N, Rocha F, Vidal EAG and Pardo-Gandarillas MC (2021) Macroevolutionary trade-offs and trends in life history traits of cephalopods through a comparative phylogenetic approach. Front Mar Sci 8:707825. https://doi.org/10.3389/fmars.2021.707825
Iwata Y, Sauer WH, Sato N, Shaw PW (2018) Spermatophore dimorphism in the chokka squid Loligo reynaudii associated with alternative mating tactics. J Molluscan Stud 84(2):157–162
Jereb P, Vecchione M, Roper CFE (2010) Family Loliginidae. In: Jereb P, Roper CFE (eds) Cephalopods of the world. An annotated and illustrated catalogue of species known to date Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes No 2, vol 2. FAO, Rome, pp 38–117
Johnsen S, Kier WM (1993) Intramuscular crossed connective tissue fibres: skeletal support in the lateral fins of squid and cuttlefish (Mollusca: Cephalopoda). J Zool, London 231:311–338
Laube I, Korntheuer H, Schwager M, Trautmann S, Rahbek C, Böhning-Gaese K (2013) Towards a more mechanistic understanding of traits and range sizes. Global Ecol Biogeogr 22(2):233–241
Luna A, Rocha F, Perales-Raya C (2021) A review of cephalopods (Phylum: Mollusca) of the Canary Current Large Marine Ecosystem (Central-East Atlantic, African coast). J Mar Biol Assoc UK 101:1–25
Luo B, Santana SE, Pang Y, Wang M, Xiao Y, Feng J (2019) Wing morphology predicts geographic range size in vespertilionid bats. Sci Rep 9:4526. https://doi.org/10.1038/s41598-019-41125-0
Martins RS, Juanicó M (2018) Biology, distribution and geographic variation of loliginid squids (Mollusca: Cephalopoda) off southwestern Atlantic. Zoologia (curitiba) 35:1–16
Moltschaniwskyj NA (1995) Multiple spawning in the tropical squid Photololigo sp.: what is the cost in somatic growth? Mar Biol 124:127–135
Muller-Karger FE, McClain CR, Richardson P (1988) The dispersal of Amazon’s water. Nature 333:56–59
Nesis KN (1980) Sepiids and Loliginids: A comparative review of the distribution and evolution of neritic cephalopods. Zool Zhurnal 59(5):677–688
Okutani T (1984) Life history of Aori-ika (Sepioteuthis lessoniana). Saibai Giken 13:69–75
Packard A (1972) Cephalopods and fish: the limits of convergence. Biol Rev 47(2):241–307
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
R Development Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rambaut A, Suchard M, Xie W, Drummond A (2014) Tracer v. 1.6. Institute of Evolutionary Biology, University of Edinburgh, U.K.
Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Rezende EL, Diniz-Filho JA (2012) Phylogenetic analyses: comparing species to infer adaptations and physiological mechanisms. Compr Physiol 2:639–674
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542
Roper CFE, Voss GL (1983) Guidelines for taxonomic descriptions of cephalopod species. Mem Mus Vic 44:48–63
Rosa R, Dierssen HM, González L, Seibel BA (2008a) Ecological biogeography of Cephalopod Molluscs in the Atlantic Ocean: historical and contemporary causes of coastal diversity patterns. Glob Ecol Biogeogr 17:600–610
Rosa R, Dierssen HM, González L, Seibel BA (2008b) Large-scale diversity patterns of cephalopods in the Atlantic open ocean and deep-sea. Ecology 89:3449–3461
Rosa R, González L, Dierssen HM, Seibel BA (2012) Environmental determinants of latitudinal size-trends in cephalopods. Mar Ecol Prog Ser 464:153–165
Rosa R, Pissarra V, Borges FO, Santos C, Xavier J, Gleadall IG, Golikov A, Bello G, Morais L, Lishchenko F, Roura Á, Judkins H, Ibáñez CM, Piatkowski U, Vecchione M, Villanueva R (2019) Global patterns of species richness in coastal cephalopods. Front Mar Scienc 6:469
Roura Á, Álvarez-Salgado XA, González ÁF, Gregori M, Rosón G, Otero J, Guerra Á (2016) Life strategies of cephalopod paralarvae in a coastal upwelling system (NW Iberian Peninsula): insights from zooplankton community and spatio-temporal analyses. Fish Oceanogr 25:241–258
Roura Á, Amor M, González ÁF, Guerra Á, Barton ED, Strugnell JM (2019) Oceanographic processes shape genetic signatures of planktonic cephalopod paralarvae in two upwelling regions. Progr Oceanogr 170:11–27
Sales JB, Shaw PW, Haimovici M, Markaida U, Cunha DB, Ready JS, Figueiredo-Ready WM, Angioletti F, Schneider H, Sampaio I (2013) New molecular phylogeny of the squids of the family Loliginidae with emphasis on the genus Doryteuthis Naef, 1912: mitochondrial and nuclear sequences indicate the presence of cryptic species in the southern Atlantic Ocean. Mol Phylogenet Evol 68(2):293–299
Sales JBL, Rodrigues-Filho LFS, Ferreira YS, Carneiro J, Asp NE, Shaw PW, Haimovici M, Markaida U, Ready J, Schneider H, Sampaio I (2017) Divergenceof cryptic species of Doryteuthis plei Blainville, 1823 (Loliginidae, Cephalopoda) in the Western Atlantic Ocean is associated with the formation of the Caribbean Sea. Mol Phylogenet Evol 106:44–54
Segawa S, Hirayama S, Okutani T (1993) Is Sepioteuthis lessoniana in Okinawa a single species? In: Okutanitodor RK, Kubodera T (eds) Recent advances in Cephalopod fisheries biology. Tokai University Press, Tokyo, pp 513–521
Shaw PW, Pierce G, Boyle PR (1999) Subtle population structuring within a highly vagile marine invertebrate, the veined squid Loligo forbesi (Cephalopoda: Loliginidae) uncovered using microsatellite DNA markers. Mol Ecol 8:407–417
Shaw PW, Hendrickson L, McKeown NJ, Stonier T, Naud MJ, Sauer WHH (2010) Discrete spawning aggregations of loliginid squid do not represent genetically distinct populations. Mar Ecol Prog Ser 408:117–127
Stewart WJ, Bartol IK, Krueger PS (2010) Hydrodynamic fin function of brief squid. Lolliguncula Brevis J Exp Biol 213(12):2009–2024
Ulloa PM, Hernández CE, Rivera RJ, Ibáñez CM (2017) Biogeografía histórica de los calamares de la familia Loliginidae (Teuthoidea: Myopsida). Lat Am J Aquat Res 45:113–129
Villanueva R, Vidal EAG, Fernández-Álvarez FÁ, Nabhitabhata J (2016) Early mode of life and hatchling size in cephalopod molluscs: influence on the species distributional ranges. PLoS ONE 11:e0165334
Voss GL (1963) Cephalopods of the Philippine Islands. Bull US Natl Mus. https://doi.org/10.5479/si.03629236.234.1
Acknowledgements
We would like to thank Francisco Rocha for the comments on the initial stages of the manuscript.
Funding
CMI acknowledges funding grant REG UNAB 04-2020. JBLS thanks Instituto de Ciencias Biologicas from UFPA for the project support number 041/2020/ICB/UFPA.
Author information
Authors and Affiliations
Contributions
CMI and CM-G were involved in conceptualization, compiled data, methodology, formal analysis, writing—original draft, and review and editing. FIT, AL, and JBLS were involved in conceptualization and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest and also no financial interests.
Ethical approval
Ethical review and approval were not required for this study because this work does not contain any experimental studies with live animals. Biological, distributional data, as well as sequences, were taken from open sources (i.e. GenBank, FAO books). This study did not need ethical approval since it was based on literature review and published information.
Additional information
Responsible Editor: R. Rosa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ibáñez, C.M., Luna, A., Márquez-Gajardo, C. et al. Biological traits as determinants in the macroecological patterns of distribution in loliginid squids. Mar Biol 170, 133 (2023). https://doi.org/10.1007/s00227-023-04286-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04286-1