Abstract
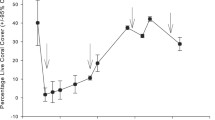
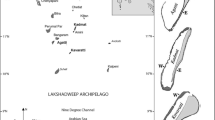
Coral reefs are experiencing a dramatic loss of hard coral abundance and associated habitat structure from a myriad of local and global factors. Here, utilizing U–Th radiometric age-dating of coral death assemblages, we investigated patterns of coral mortality from the eastern margin of the Red Sea along a latitudinal gradient (Yanbu, 24o N; Thuwal, 22o N; Al-Lith, 19o N; Farasan Banks, 18o N) in 2018 and 2019. In all four regions, radiometric ages of in situ dead Acropora and Pocillopora colonies were largely confined to the late twentieth and early twenty-first century. During the early twenty-first century, coral mortality was found to be synchronous with previously documented bleaching events in 2010 and 2015 and, at one site (Farasan Banks), an outbreak of crown-of-thorns starfish (COTS) in 2009. The most northern site, Yanbu, had the highest relative percentage of live coral (42 ± 4%) and of living Acropora, and may serve as a refugium under climate warming scenarios. For the three southern regions (Thuwal, Al-Lith, Farasan Banks) benthic structure was mostly comprised of dead corals. The southernmost survey site, Farasan Banks, underwent a dramatic change in coral benthic structure associated with a COTS outbreak in 2009 and a bleaching event in 2015, and had the lowest relative percentage of live coral (6 ± 2%), comprised mostly of massive Porites, with no live Acropora or Pocillopora. Our results highlight the asynchronous impact of disturbance events on eastern Red Sea coral reefs and emphasize regional differences in recovery and ecosystem state.







Similar content being viewed by others
Availability of data and material
The U–Th radiometric age-dates and ecological data (coral life and death assemblages) for this study are included as supplementary tables.
Code availability
The R code was written and executed within R Studio and will be provided through a request to the corresponding author.
References
Agulles M, Jordà G, Jones B, Agusti S, Duarte CM (2019) Temporal evolution of Red Sea temperatures based on in-situ observations (1958–2017). Ocean Sci 16:149–166
Anton A, Randle JL, Garcia FC, Rossbach S, Ellis JI, Weinzierl M, Duarte CM (2020) Differential thermal tolerance between algae and corals may trigger the proliferation of algae in coral reefs. Glob Chang Biol 26:4316–4327
Berumen ML, Voolstra CR, Daffonchio D, Agusti S, Aranda M, Irigoien X, Jones BH, Moran XAG, Duarte CM (2019) The Red Sea: Environmental gradients shape a natural laboratory in a nascent ocean. In: Voolstra C, Berumen M (eds) Coral reefs of the Red Sea, coral reefs of the world 11. Springer, Cham. https://doi.org/10.1007/978-3-030-05802-9_1
Bruckner AW, Dempsey AC (2015) The status, threats, and resilience of reef-building corals of the Saudi Arabian Red Sea. In: Rasul N, Stewart I (eds) The Red Sea. Springer earth system sciences. . Springer, Berlin. https://doi.org/10.1007/978-3-662-45201-1_27
Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. Washington (USA): World Resources Institute, 130 pp. http://www.wri.org/publication/reefs-risk-revisited
Cantin NE, Cohen AL, Karnauskas KB, Tarrant AM, McCorkle DC (2010) Ocean warming slows coral growth in the central Red Sea. Science 329:322–325
Chaidez VD, Dreano D, Agusti S, Duarte CM, Hoteit I (2017) Decadal trends in Red Sea maximum surface temperature. Sci Rep 7:8144
Churchill JH, Bower AS, McCorkle DC, Abualnaja Y (2014) The transport of nutrient-rich Indian Ocean water through the Red Sea and into coastal reef system. J Mar Res 72:165–181
Claar DC, Szostek L, McDevitt-Irwin JM, Schanze JJ, Baum JK (2018) Global patterns and impacts of El Niño events on coral reefs: a meta-analysis. PLoS ONE 13:e0190957
Clark TR, Zhao J, Feng Y, Done TJ, Jupiter S, Lough J, Pandolfi JM (2012) Spatial variability of initial 230Th/232Th in modern Porites from the inshore region of the Great Barrier Reef. Geochim Cosmochim Acta 78:99–118
Clark TR, Zhao J, Roff G, Feng Y, Done TJ, Nothdurft LD, Pandolfi JM (2014) Discerning the timing and cause of historical mortality events in modern Porites from the Great Barrier Reef. Geochim Cosmochim Acta 138:57–80
Clark TR, Roff G, Zhao J, Feng Y, Done TJ, McCook LJ, Pandolfi JM (2017) U-Th dating reveals regional-scale decline of branching Acropora corals on the Great Barrier Reef over the past century. PNAS 114:10350–10355
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
D’Olivo JP, Georgiou L, Falter J, DeCarlo TM, Irigoien X, Voolstra CR, Roder C, Trotter J, McCulloch MT (2019) Long-term impacts of the 1997–1998 bleaching event on the growth and resilience of massive Porites corals from the central Red Sea. Geochem Geophys Geosys 20:2936–2954
DeCarlo TM, Gajdzik L, Ellis J, Coker DJ, Roberts MB, Hammerman NM, Pandolfi JM, Monroe AA, Berumen ML (2020) Nutrient-supplying ocean currents modulate coral bleaching susceptibility. Sci Adv 6:34
DeVantier L, Turak E, Al-Shaikh K, De’ath G (2000) Coral communities of the central-northern Saudi Arabian Red Sea. Fauna Arabia 18:23–66
Duarte CM, Agusti S, Barbier E, Britten GL, Castilla JC, Gattuso JP, Fulweiler RW, Hughes TP, Knowlton N, Lovelock CE, Lotze HK, Predragovic M, Poloczanska E, Roberts C, Worm B (2020) Rebuilding marine life. Nature 580:39–51
Eakin CM, Sweatman HPA, Brainard RE (2019) The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38:539–545
Ellis JI, Jamil T, Anlauf H, Coker DJ, Curdia J, Hewitt J, Jone BH, Krokos G, Kurten B, Hariprasad D, Roth F (2019) Multiple stressor effects on coral reef ecosystems. Glob Chang Biol 25:4132–4146
Fine M, Gildor H, Genin A (2013) A coral reef refuge in the Red Sea. Glob Chang Biol 19:3640–3647
Furby KA, Bouwmeester J, Berumen ML (2013) Susceptibility of central Red Sea corals during a major bleaching event. Coral Reefs 32:505–513
Gegner HM, Radecker N, Ochsenkuhn M, Barreto MM, Ziegler M, Reichert J, Schubert P, Wilke T, Voolstra CR (2019) High levels of floridoside at high salinity link osmoadaptation with bleaching susceptibility in the cnidarian-algal endosymbiosis. Biol Open 8:bio045591
Genevier LGC, Jamil T, Raitsos DE, Krokos G, Hoteit I (2019) Marine heatwaves reveal coral reef zones susceptible to bleaching in the Red Sea. Glob Chang Biol 25:2338–2351
Greenstein BJ, Curran HA, Pandolfi JM (1998) Shifting ecological baselines and the demise of Acropora cervicornis in the western North Atlantic and Caribbean Province: a Pleistocene perspective. Coral Reefs 17:249–261
Grottoli AG, Tchernov D, Winters G (2017) Physiological and biogeochemical responses of super-corals to thermal stress from the Northern Gulf of Aqaba Red Sea. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00215
Hariri MSB (2012) The present status of the Red Sea coral reefs between Haql and Yanbu, Saudi Arabia. Life Sci 9:3852–3859
Hoaglin DC, Mosteller F, Tukey JW (1983) Understanding robust and exploratory data analysis. Wiley-Interscience, New York, NY
Hoegh-Guldberg O, Poloczanska ES, Skirving W, Dove S (2017) Coral reef ecosystems under climate change and ocean acidification. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00158
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (2012) Assembly rules of reef corals are flexible along a steep climatic gradient. Curr Biol 22:736–741
Hughes TP, Kerry JT, Alvarez-Noriega M et al (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK et al (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Ivchenko GI, Honov SA (1998) On the Jaccard similarity test. J Math Sci 88:789–794
Khalil MT, Bouwmeester J, Berumen ML (2017) Spatial variation in coral reef fish and benthic communities in the central Saudi Arabian Red Sea. PeerJ 5:e3410
Kidwell SM (2007) Discordance between living and death assemblages as evidence for anthropogenic ecological change. PNAS 104:17701–17706
Kleypas JA, Danabasoglu G, Lough JM (2008) Potential role of the ocean thermostat in determining regional differences in coral reef bleaching events. Geophys Res Lett 35:GL032257
Kohler KE, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269
Kruskal JB (1964) Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–17
Liu G, Heron SF, Eakin CM, Muller-Karger FE, Vega-Rodriguez M, Guild LS, De La Cour JL, Geiger EF, Skirving WJ, Burgess TFR, Strong AE, Harris A, Maturi E, Ignatov A, Sapper J, Li J, Lynds S (2014) Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA Coral Reef Watch. Remote Sensing 11:11579–11606
MacNeil MA, Graham NAJ, Cinner JE, Wilson SK, Williams ID, Maina J, Newman S, Friedlander A, Jupiter S, Polunin NVC, McClanahan TR (2015) Recovery potential of the world’s coral reef fishes. Nature 520:341–344
Martins I, Oliveira JM, Flindt MR, Marques JC (1999) The effect of salinity on the growth rate of the macroalgae Enteromorpha intestinalis (Chlorophyta) in the Mondego estuary (west Portugal). Acta Oecologica 20:259–265
Monroe AA, Ziegler M, Roik Z, Rothig T, Hardenstine RS, Emms MA, Jensen T, Voolstra CR, Berumen ML (2018) In situ observations of coral bleaching in the central Saudi Arabian Red Sea during the 2015/2016 global coral bleaching event. PLoS ONE 13:e0195814
Morais J, Medeiros APM, Santos BA (2018) Research gaps of coral ecology in a changing world. Mar Environ Res 140:243–250
Moustafa MZ, Moustafa ZD, Moustafa MS (2013) Resilience of a high latitude Red Sea corals to extreme temperature. Open J Ecol 3:242–253
Nyström M, Graham NAJ, Lokrantz J, Norström AV (2008) Capturing the cornerstones of coral reef resilience: linking theory to practice. Coral Reefs 27:795–809
Obura DO, Grimsditch GD (2009) Resilience assessment of coral reefs: Rapid assessment protocol for coral reefs, focusing on coral bleaching and thermal stress. IUCN working group on Climate Change and Coral Reefs. IUCN, Gland, Switzerland. 70 pages
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) vegan: Community Ecology Package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan
Osman EO, Smith DJ, Ziegler M, Kürten B, Conrad C, El-Haddad KM, Voolstra CR, Suggett DJ (2018) Thermal refugia against coral bleaching throughout the northern Red Sea. Glob Chang Biol 24:474–484
Pandolfi JM (2015) Incorporating uncertainty in predicting the future response of coral reefs to climate change. Annu Rev Ecol Evol Syst 46:281–303
Pandolfi JM, Greenstein BJ (1997) Preservation of community structure in death assemblages of deep-water Caribbean reef corals. Limnol Oceanogr 42:1505–1516
Pandolfi JM, Minchin PR (1995) A comparison of taxonomic composition and diversity between reef coral life and death assemblages in Madang Lagoon, Papua New Guinea. Paleogeogr Palaeoclimatol Palaeoecol 119:321–341
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333:418–422
Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ, Brander K, Bruno JF, Buckley LB, Burrows MT, Duarte CM, Halpern BS, Holding J, Kappel CV, O’Connor MI, Pandolfi JM, Parmesan C, Schwing F, Thompson SA, Richardson AJ (2013) Global imprint of climate change on marine life. Nat Clim Chang 3:919–925
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/
Rahmani MR, Rahimian H, Ardalan M, Keshavmurthy S, Fontana S, Wallace CC, Chen CA (2013) Acropora distribution patterns in the northern and northeastern Persian Gulf. Zool Stud 52:40
Reynolds RW, Rayner NA, Smith TM, Stokes DC, Wang W (2002) An improved in situ and satellite SST analysis for climate. J Clim 15:1609–1625
Riegl BM, Bruckner AW, Rowlands GP, Purkis SJ, Renaud P (2012) Red Sea coral Reef Trajectories over 2 decades suggest increasing community homogenization and decline in coral size. PLoS ONE 7:e38396
Riegl BM, Berumen M, Bruckner A (2013) Coral population trajectories, increased disturbance and management intervention: a sensitivity analysis. Ecol Evol 3:1050–1064
Roberts MB, Jones GP, McCormick MI, Munday PL, Neale S, Thorrold S, Robitzch VSN, Berumen ML (2016) Homogeneity of coral reef communities across 8 degrees of latitude in the Saudi Arabian Red Sea. Mar Pollut Bull 105:558–565
Roff G, Clark TR, Reymond CE, Zhao J, Feng Y, McCook LJ, Done TJ, Pandolfi JM (2013) Paleoecological evidence of a historical collapse of corals at Pelorus Island, inshore Great Barrier Reef, following European settlement. Proc Royal Soc B 280:20122100
Rowlands G, Purkis S, Riegl B, Metsamaa L, Bruckner A, Renaud P (2012) Satellite imaging coral reef resilience at regional scale. A case-study form Saudi Arabia. Mar Pollut Bull 64:1222–1237
Sheppard CR, Price A, Roberts C (1992) Marine ecology of the Arabian region: patterns and processes in extreme tropical environments. Academic Press, Harcourt Brace Jovanovich, London
Sheppard CR, Sheppard ALS (1991) Corals and coral communities of Arabia. In: Buttiker W,Krupp F (eds) Fauna of Saudi Arabia 12, Natural History Museum, Basle, pp 3–170
Sofianos SS, Johns WE (2003) An oceanic general circulation model (OGCM) investigation of the Red Sea circulation: 2. Three-dimensional circulation in the Red Sea. J Geophys Res 108:3066
Sully S, Burkepile DE, Donovan MK, Hodgson G, van Woesik R (2019) A global analysis of coral bleaching over the past two decades. Nat Commun 10:1264
Trommer G, Siccha M, Rohling EJ, Grant K, van der Meer MTJ, Schouten S, Hemleben C, Kucera M (2010) Millennial-scale variability in Red Sea circulation in response to Holocene insolation forcing. Paleoceanogr Paleoclimatology 25:PA3203
Vercelloni J, Liquet B, Kennedy EV, González-Rivero M, Caley MJ, Peterson EE, Puotinen M, Hoegh-Guldberg O, Mengersen K (2020) Forecasting intensifying disturbance effects on coral reefs. Glob Chang Biol 26:2785–2797
Vermeesch P (2018) IsoplotR: a free and open toolbox for geochronology. Geosci Front 9:1479–1493
Zhao J, Yu K, Feng Y (2009) High-precision 238U–234U- 230Th disequilibrium dating of the recent past: a review. Quat Geochronol 4:423–443
Acknowledgements
This research was supported by funding from a collaborative subaward agreement between the King Abdullah University of Science and Technology (KAUST) and The University of Queensland (UQ) awarded to J.M.P. and C.M.D. (OSR-2018-CARF-1973-03) and the Australian Research Council Centre of Excellence for Coral Reef Studies grant to J.M.P. and others (CE140100020). We thank the crew and technicians aboard the R/V Thuwal stationed at KAUST for our first field excursion in Saudi Arabia; K. Rowe, S. Persson and A. Gusti for help in the field for both trips; A. Nguyen, Y. Feng, and F. Liu from the Radiogenic Isotope Facility (UQ); G. Xia from the Earth Science Sample Preparation Laboratory (UQ); and K. Zwiep, S. Kim, K. Gomez-Cabrera, C. Chong-Montenegro, H. Markham, and C. Sims from the Marine Palaeoecology Lab (UQ). N.M.H. was supported with a University of Queensland Research Training Scholarship. We also thank the reviewers for their contributions in reviewing this manuscript.
Funding
This research was supported by funding from a collaborative subaward agreement between the King Abdullah University of Science and Technology (KAUST) and The University of Queensland (UQ) awarded to J.M.P. and C.M.D. (OSR-2018-CARF-1973–03) and the Australian Research Council Centre of Excellence for Coral Reef Studies grant to JMP and others (CE140100020). All applicable international, national, and institutional guidelines for sampling, care, and experimental use of organisms for this study have been followed and all necessary approvals have been obtained.
Author information
Authors and Affiliations
Contributions
NMH, ARR, GR, and JMP conceived and designed the study. NMH led the study, data analysis, interpretation and manuscript writing with input from all authors. NMH, ARR, SR, MNH, and VFS conducted the fieldwork. NMH, ARR, NL, and JxZ performed laboratory analyses. TLS, NL, GR, and TMD helped with data manipulation, visualization, and analysis. CMD and JMP provided funding and mentorship.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
We, the co-authors, consent for this manuscript to be published in Marine Biology and confirm it is not in submission or publication elsewhere.
Additional information
Responsible Editor: S. Harii.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
227_2021_3984_MOESM1_ESM.pdf
Fig. S1 Representative quadrate photographs of modern assemblages from each region and site (Photo credit: Nicholas M. Hammerman). Fig. S2 Representative photographs of the in-growth position colonies taken for radiometric (U–Th) age-dating at each region and site (Photo credit: Nicholas M. Hammerman). Fig. S3 Photographs of massive Porites colonies sampled for radiometric (U–Th) age-dating from Thuwal, note pick = 25 cm (Photo credit: Nicholas M. Hammerman). Fig. S4 Non-metric multi-dimensional scaling (nMDS) ordination of coral families a) life, and b) death assemblages from reefs sampled from four regions of the Saudi Arabian Red Sea using relative abundance data (dotted lines are 95% confidence ellipses), highlighting the differences in live and dead coral among the four regions. Each symbol represents a separate transect. Overlaid vectors are the weighted contribution of each family’s relative abundance (PDF 659 KB)
Rights and permissions
About this article
Cite this article
Hammerman, N.M., Rodriguez-Ramirez, A., Staples, T.L. et al. Variable response of Red Sea coral communities to recent disturbance events along a latitudinal gradient. Mar Biol 168, 177 (2021). https://doi.org/10.1007/s00227-021-03984-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-03984-y