Abstract
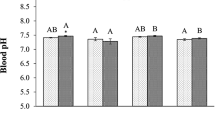
Ocean acidification, a reduction in the pH of the oceans caused by increasing CO2, can have negative physiological effects on marine species. In this study, we examined how CO2-driven acidification affected the growth and survival of juvenile golden king crab (Lithodes aequispinus), an important fishery species in Alaska. Juveniles were reared from larvae in surface ambient pH seawater at the Kodiak Laboratory. Newly molted early benthic instar crabs were randomly assigned to one of three pH treatments: (1) surface ambient pH ~ 8.2, (2) likely in situ ambient pH 7.8, and (3) pH 7.5. Thirty crabs were held in individual cells in each treatment for 127 days and checked daily for molting or death. Molts and dead crabs were photographed under a microscope and measured using image analysis to assess growth and morphology. Mortality was primarily associated with molting in all treatments, differed among all treatments, and was highest at pH 7.5 and lowest at ambient pH. Crabs at pH 7.5 were smaller than crabs at ambient pH at the end of the experiment, both in terms of carapace length and wet mass; had a smaller growth increment after molting; had a longer intermolt period. Carapace morphology was not affected by pH treatment. Decreased growth and increased mortality in laboratory experiments suggest that lower pH could affect golden king crab stocks and fisheries. Future work should examine if larval rearing conditions affect the juvenile response to low pH.






Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study included as a supplement to this paper.
Code availability
The code generated during the current study is available from the corresponding author on reasonable request.
References
Agnalt AL, Grefsrud ES, Farestveit E, Larsen M, Keulder F (2013) Deformities in larvae and juvenile European lobster (Homarus gammarus) exposed to lower pH at two different temperatures. Biogeosciences 10:7883–7895. https://doi.org/10.5194/bg-10-7883-2013
Appelhans YS, Thomsen J, Pansch C, Melzner F, Wahl M (2012) Sour times: seawater acidification effects on growth, feeding behaviour and acid-base status of Asterias rubens and Carcinus maenas. Mar Ecol Progr Ser 459:85–98. https://doi.org/10.3354/meps09697
Blau SF, Pengilly D, Tracy DA (1996) Distribution of golden king crabs by sex, size, and depth zones in the Eastern Aleutian Islands, Alaska. In: Baxter B, Donaldson WE, Paul AJ, Otto RS, Witherell DB (eds) High latitude crabs: biology. Management and Economics Alaska Sea Grant College Program, University of Alaska Fairbanks, Anchorage, AK, pp 167–185
Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP, Gehlen M, Halloran P, Heinze C, Ilyina T, Seferian R (2013) Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10:6225–6245. https://doi.org/10.5194/bg-10-6225-2013
Breitburg DL, Salisbury J, Bernhard JM, Cai W-J, Dupont S, Doney SC, Kroeker KJ, Levin LA, Long WC, Milke LM (2015) And on top of all that… Coping with ocean acidification in the midst of many stressors. Oceanography 28:48–61. https://doi.org/10.5670/oceanog.2015.31
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Byrne RH, Mecking S, Feely RA, Liu X (2010) Direct observations of basin-wide acidification of the North Pacific Ocean. Geophys Res Lett 37:L02601
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365. https://doi.org/10.1038/425365a
Chang ES (1995) Physiological and biochemical changes during the molt cycle in decapod crustaceans: an overview. J Exp Mar Biol Ecol 193:1–14
Coffey WD, Yarram A, Matoke B, Long WC, Swiney KM, Foy RJ, Dickenson G (2017) Ocean acidification leads to altered micromechanical properties of the mineralized cuticle of juvenile red and blue king crabs. J Exp Mar Biol Ecol 495:1–12. https://doi.org/10.1016/j.jembe.2017.05.011
Cross JN, Mathis JT, Bates NR, Byrne RH (2013) Conservative and non-conservative variations of total alkalinity on the southeastern Bering Sea shelf. Mar Chem 154:100–112. https://doi.org/10.1016/j.marchem.2013.05.012
deVries MS, Webb SJ, Tu J, Cory E, Morgan V, Sah RL, Deheyn DD, Taylor JRA (2016) Stress physiology and weapon integrity of intertidal mantis shrimp under future ocean conditions. Sci Rep 6:15. https://doi.org/10.1038/srep38637
Dissanayake A, Ishimatsu A (2011) Synergistic effects of elevated CO2 and temperature on the metabolic scope and activity in a shallow-water coastal decapod (Metapenaeus joyneri; Crustacea: Penaeidae). ICES J Mar Sci 68:1147–1154. https://doi.org/10.1093/icesjms/fsq188
DOE (1994) Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water. Version 2. U.S. Department of Energy
Donaldson W, Byersdorfer S (2005) Biological field techniques for lithodid crabs. University of Alaska Fairbanks, Fairbanks, AK, Alaska Sea Grant College Program
Dupont S, Dorey N, Stumpp M, Melzner F, Thorndyke M (2013) Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar Biol 160:1835–1843. https://doi.org/10.1007/s00227-012-1921-x
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsn048
Fabry VJ, McClintock JB, Mathis JT, Grebmeier JM (2009) Ocean acidification at high latitudes: the bellwether. Oceanography 22:160–171
Garber-Yonts B, Lee J (2013) Stock Assessment and Fishery Evaluation Report for King and Tanner Crab Fisheries of the Bering Sea and Aleutian Islands Regions: Economic Status of the BSAI Crab Fisheries, 2013. National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Alaska Fisheries Science Center, Seattle, Washington
Glandon HL, Miller TJ (2017) No effect of high pCO2 on juvenile blue crab, Callinectes sapidus, growth and consumption despite positive responses to concurrent warming. ICES J Mar Sci 74:1201–1209. https://doi.org/10.1093/icesjms/fsw171
Glandon HL, Paynter KT, Rowe CL, Miller TJ (2019) Resilience of oxygen consumption rates in the juvenile blue crab Callinectes sapidus to future predicted increases in environmental temperature and pCO2 in the mesohaline Chesapeake Bay. J Shellfish Res 38:711–723. https://doi.org/10.2983/035.038.0323
Hans S, Fehsenfeld S, Treberg JR, Weihrauch D (2014) Acid-base regulation in the Dungeness crab (Metacarcinus magister). Mar Biol 161:1179–1193. https://doi.org/10.1007/s00227-014-2409-7
Heinrich L, Krause T (2017) Fishing in acid waters: a vulnerability assessment of the Norwegian fishing industry in the face of increasing ocean acidification. Integr Environ Assess Manage 13:778–789. https://doi.org/10.1002/ieam.1843
IPCC (2014) Climate Change 2014 Fifth Assessment Synthesis Report
Johnson EG, Hines AH, Kramer MA, Young AC (2008) Importance of season and size of release to stocking success for the blue crab in Chesapeake Bay. Rev Fish Sci 16:243–253
Kelly MW, Padilla-Gamino JL, Hofmann GE (2013) Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Global Chang Biol 19:2536–2546. https://doi.org/10.1111/gcb.12251
Knapp JL, Bridges CR, Krohn J, Hoffman LC, Auerswald L (2016) The effects of hypercapnia on the West Coast rock lobster (Jasus lalandii) through acute exposure to decreased seawater pH—physiological and biochemical responses. J Exp Mar Biol Ecol 476:58–64. https://doi.org/10.1016/j.jembe.2015.12.001
Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Chang Biol 19:1884–1896
Kurihara H, Matsui M, Furukawa H, Hayashi M, Ishimatsu A (2008) Long-term effects of predicted future seawater CO2 conditions on the survival and growth of the marine shrimp Palaemon pacificus. J Exp Mar Biol Ecol 367:41–46. https://doi.org/10.1016/j.jembe.2008.08.016
Langer JAF, Meunier CL, Ecker U, Horn HG, Schwenk K, Boersma M (2019) Acclimation and adaptation of the coastal calanoid copepod Acartia tonsa to ocean acidification: a long-term laboratory investigation. Mar Ecol Progr Ser 619:35–51. https://doi.org/10.3354/meps12950
Lavigne H, Gattuse J (2012) seacarb: Seawater carbonate chemistry with R. http://CRAN.R-project.org/package=seacarb
Lee YH, Jeong CB, Wang MH, Hagiwara A, Lee JS (2020) Transgenerational acclimation to changes in ocean acidification in marine invertebrates. Mar Pollut Bull 153:18. https://doi.org/10.1016/j.marpolbul.2020.111006
Long WC, Swiney KM, Foy RJ (2013a) Effects of ocean acidification on the embryos and larvae of red king crab, Paralithodes camtschaticus. Mar Pollut Bull 69:38–47. https://doi.org/10.1016/j.marpolbul.2013.01.011
Long WC, Swiney KM, Harris C, Page HN, Foy RJ (2013b) Effects of ocean acidification on juvenile red king crab (Paralithodes camtschaticus) and Tanner crab (Chionoecetes bairdi) growth, condition, calcification, and survival. PLoS ONE 8:e60959. https://doi.org/10.1371/journal.pone.0060959
Long WC, Van Sant SB, Swiney KM, Foy R (2017) Survival, growth, and morphology of blue king crabs: effect of ocean acidification decreases with exposure time. ICES J Mar Sci 74:1033–1041. https://doi.org/10.1093/icesjms/fsw197
Long WC, Cummiskey P, Munk JE (2018) How does stocking density affect enhancement success for hatchery-reared red king crab? Can J Fish Aquat Sci 75:1940–1948. https://doi.org/10.1139/cjfas-2017-0330
Long WC, Pruisner P, Swiney KM, Foy R (2019) Effects of ocean acidification on respiration, feeding, and growth of juvenile red and blue king crabs (Paralithodes camtschaticus and P. platypus). ICES J Mar Sci 76:1335–1343. https://doi.org/10.1093/icesjms/fsz090
Mangum C, McMahon B, Defur P, Wheatly M (1985) Gas exchange, acid-base balance, and the oxygen supply to the tissues during a molt of the blue crab Callinectes sapidus. J Crust Biol 5:188–206
Mathis JT, Cross JN, Evans W, Doney SC (2015) Ocean acidification in the surface waters of the Pacific-Arctic boundary regions. Oceanography 28:122–135. https://doi.org/10.5670/oceanog.2015.36
McLean EL, Katenka NV, Seibel BA (2018) Decreased growth and increased shell disease in early benthic phase Homarus americanus in response to elevated CO2. Mar Ecol Prog Ser 596:113–126
Menu-Courey K, Noisette F, Piedalue S, Daoud D, Blair T, Blier PU, Azetsu-Scott K, Calosi P (2019) Energy metabolism and survival of the juvenile recruits of the American lobster (Homarus americanus) exposed to a gradient of elevated seawater pCO2. Mar Environ Res 143:111–123. https://doi.org/10.1016/j.marenvres.2018.10.002
Pane EF, Barry JP (2007) Extracellular acid-base regulation during short-term hypercapnia is effective in a shallow-water crab, but ineffective in a deep-sea crab. Mar Ecol Prog Ser 334:1–9. https://doi.org/10.3354/meps334001
Parker LM, Ross PM, O’Connor WA, Borysko L, Raftos DA, Pörtner HO (2012) Adult exposure influences offspring response to ocean acidification in oysters. Global Chang Biol 18:82–92
Parker LM, O’Connor WA, Raftos DA, Pörtner H-O, Ross PM (2015) Persistence of positive carryover effects in the oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS ONE 10:e0132276. https://doi.org/10.1371/journal.pone.0132276
Pirtle JL, Eckert GL, Stoner AW (2012) Habitat structure influences the survival and predator-prey interactions of early juvenile red king crab Paralithodes camtschaticus. Mar Ecol Prog Ser 465:169–184. https://doi.org/10.3354/meps09883
Punt AE, Foy RJ, Dalton MG, Long WC, Swiney KM (2016) Effects of long term exposure to ocean acidification on future southern Tanner crab (Chionoecetes bairdi) fisheries management. ICES J Mar Sci 73:849–864
Punt AE, Dalton MG, Foy RJ (2020) Multispecies yield and profit when exploitation rates vary spatially including the impact on mortality of ocean acidification on North Pacific crab stocks. Fish Res 225:18. https://doi.org/10.1016/j.fishres.2019.105481
Roberts JL (1957) Thermal acclimation of metabolism in the crab Pachygrapsus crassipes Randall. I. The influence of body size, starvation, and molting. Physiol Zool 30:232–242
Shirley TC (2006) Cultivation potential of golden king crab, Lithodes aequispinus. In: Stevens BG (ed) Alaska Crab Stock Enhancement and Rehabilitation. Alaska Sea Grant College Program, Kodiak, Alaska, pp 47–54
Shirley T, Zhou S (1997) Lecithotrophic development of the golden king crab Lithodes aequispinus (Anomura: Lithodidae). J Crust Biol 17:207–216
Sloan N (1985) Life history characteristics of fjord-dwelling golden king crabs Lithodes aequispina. Mar Ecol Progr Ser 22:219–228
Small DP, Calosi P, Boothroyd D, Widdicombe S, Spicer JI (2016) The sensitivity of the early benthic juvenile stage of the European lobster Homarus gammarus (L.) to elevated pCO2 and temperature. Mar Biol 163:1–12
Swiney KM, Long WC, Persselin SL (2013) The effects of holding space on juvenile red king crab (Paralithodes camtschaticus) growth and survival. Aquacult Res 44:1007–1016. https://doi.org/10.1111/j.1365-2109.2012.03105.x
Swiney KM, Long WC, Foy RJ (2016) Effects of high pCO2 on Tanner crab reproduction and early life history- Part I: long-term exposure reduces hatching success and female calcification, and alters embryonic development. ICES J Mar Sci 73: 825–835
Swiney KM, Long WC, Foy RJ (2017) Decreased pH and increased temperatures affect young-of-the-year red king crab (Paralithodes camtschaticus). ICES J Mar Sci 74:1191–1200. https://doi.org/10.1093/icesjms/fsw251
Tanner CA, Burnett LE, Burnett KG (2006) The effects of hypoxia and pH on phenoloxidase activity in the Atlantic blue crab, Callinectes sapidus. Comp Biochem Physiol: Mol Integr Physiol 144:218–223
Tarverdieva MI, Zgurovsky KA (1985) On food composition of the deep-water crab species Lithodes aequispina Benedict and Chionoecetes tanneri Rathbun in the Bering Sea and Okhotsk seas. In: Davis SK, Gaffney F, McCrary J, Paul AJ, Otto RS (eds) Proceedings of the International King Crab Symposium. Alaska Sea Grant College Program, University of Alaska Fairbanks, Anchorage, AK, pp 319–329
Thor P, Dupont S (2015) Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Global Chang Biol 21:2261–2271. https://doi.org/10.1111/gcb.12815
Thor P, Bailey A, Dupont S, Calosi P, Soreide JE, De Wit P, Guscelli E, Loubet-Sartrou L, Deichmann IM, Candee MM, Svensen C, King AL, Bellerby RGJ (2018) Contrasting physiological responses to future ocean acidification among Arctic copepod populations. Global Chang Biol 24:E365–E377. https://doi.org/10.1111/gcb.13870
Turra A, Ragagnin MN, McCarthy ID, Fernandez WS (2020) The effect of ocean acidification on the intertidal hermit crab Pagurus criniticornis is not modulated by cheliped amputation and sex. Mar Environ Res 153:10. https://doi.org/10.1016/j.marenvres.2019.104794
Walther K, Anger K, Portner HO (2010) Effects of ocean acidification and warming on the larval development of the spider crab Hyas araneus from different latitudes (54 degrees vs. 79 degrees N). Mar Ecol Progr Ser 417:159–170. https://doi.org/10.3354/meps08807
Zhao LQ, Yang F, Milano S, Han TK, Walliser EO, Schone BR (2018) Transgenerational acclimation to seawater acidification in the Manila clam Ruditapes philippinarum: Preferential uptake of metabolic carbon. Sci Total Environ 627:95–103. https://doi.org/10.1016/j.scitotenv.2018.01.225
Zhao L, Liu B, An W, Deng Y, Lu Y, Liu B, Wang L, Cong Y, Sun X (2019) Assessing the impact of elevated pCO2 within and across generations in a highly invasive fouling mussel (Musculista senhousia). Sci Total Environ 689:322–331. https://doi.org/10.1016/j.scitotenv.2019.06.466
Acknowledgements
This work was funded through the NOAA Ocean Acidification Program. We thank B. Daly for rearing the juvenile crab and staff of the Kodiak Laboratory for help performing the experiments. Previous versions of this paper were improved by comments from E. Fedwa and J. Long and two anonymous reviewers. The findings and conclusions in the paper are those of the authors and do not necessarily represent the views of the National Marine Fisheries Service, NOAA. Reference to trade names or commercial firms does not imply endorsement by the National Marine Fisheries Service, NOAA.
Funding
This work was funded through the NOAA Ocean Acidification Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors affirm that they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: H.-O. Pörtner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewers: undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Long, W.C., Swiney, K.M. & Foy, R.J. Effects of ocean acidification on young-of-the-year golden king crab (Lithodes aequispinus) survival and growth. Mar Biol 168, 126 (2021). https://doi.org/10.1007/s00227-021-03930-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-03930-y