Abstract
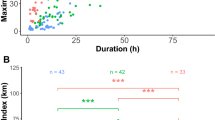
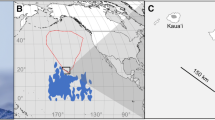
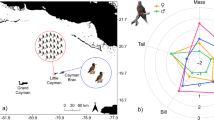
Prions Pachyptila are the most abundant seabirds in the Southern Ocean and comprise two main groups: those with and without bill lamellae to filter zooplankton. With few exceptions, each breeding location supports at most one species from each of these groups. However, Gough Island supports two morphologically very similar, filter-feeding species: broad-billed P. vittata and MacGillivray’s prions P. macgillivrayi. To understand how these two species co-occur in sympatry, we compared the foraging ranges, habitat selectivity, trophic segregation and moult schedules of these species using combined geolocation-immersion loggers. After breeding, both species showed a well-defined westward migration prior to moulting. Moult lasted 11–19 weeks and was significantly longer in MacGillivray’s than broad-billed prions. Moulting birds occurred in specific areas within the Argentine Basin, with little overlap between the two species. Habitat analysis revealed species-specific preferences, in particular sea surface temperature. Activity patterns also differed; MacGillivray’s prions spent more time in flight, which indicates a more active foraging strategy, relying less on filter feeding. Stable isotope ratios (δ15N) in flight feathers were greater in MacGillivray’s prion, which is consistent with its less specialized bill morphology resulting in feeding at a higher trophic level. Inter-specific spatial segregation was observed for most of the tracking period, in large part because broad-billed prions breed roughly 3 months earlier than MacGillivray’s prions. At Tristan da Cunha, 250 km farther north, where only broad-billed prions breed, they departed, moulted and returned significantly later (15–17 days) than conspecifics from Gough Island, providing evidence for character displacement in sympatry with MacGillivray’s prion.





Similar content being viewed by others
References
Amante C, Eakins BW (2009) ETOPO1 1 Arc-Minute Global Relief Model: Procedures, Data Sources and Analysis. NOAA Technical Memorandum NESDIS NGDC-24 https://www.ngdc.noaa.gov/mgg/global/relief/ETOPO1/docs/ETOPO1. Accessed 24 Oct 2016
Baylis AMM, Tierney M, Orben RA, Warwick-Evans V, Wakefield E, Grecian WJ et al (2019) Important at-sea areas of colonial breeding marine predators on the southern patagonian shelf. Sci Rep 9:8517
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458
BirdLife International (2017) Species factsheet: Pachyptila macgillivrayi. Available at: http://datazone.birdlife.org/species/factsheet/macgillivrays-prion-pachyptila-macgillivrayi. Accessed 23 Apr 2017
Bodey TW, Cleasby IR, Bell F, Parr N, Schultz A, Votier SC et al (2018) A phylogenetically controlled meta-analysis of biologging device effects on birds: deleterious effects and a call for more standardized reporting of study data. Methods Ecol Evol 9:946–955. https://doi.org/10.1111/2041-210X.12934
Bost CA, Cotté C, Bailleul F, Cherel Y, Charrassin JB, Guinet C, Ainley DG, Weimerskirch H (2009) The importance of oceanographic fronts to marine birds and mammals of the southern oceans. ICES J Mar Sci 78:363–376
Boyce MS, McDonald LL (1999) Relating populations to habitats using resource selection functions. Trend Ecol Evo 14:268–272
Bretagnolle V, Zotier R, Jouventin P (1990) Comparative population biology of four prions (genus Pachyptila) from the Indian Ocean and consequences for their taxonomic status. Auk 107:305–316
Bridge ES (2006) Influences of morphology and behaviour on wing-moult strategies in seabirds. Mar Ornithol 34:7–19
Brooke M (2004) Albatrosses and petrels across the world. Oxford University Press, Oxford
Brown WL, Wilson EO (1956) Character displacement. Syst Zool 5:49–64
Brown RM, Techow NMSM, Wood AG, Phillips RA (2015) Hybridization and back-crossing in giant petrels (Macronectes giganteus and M. halli) at Bird Island South Georgia, and a summary of hyrbridization in seabirds. PLoS One 10:e0121688
Catry T, Ramos JA, LeCorre M, Phillips RA (2009) Movements, at- sea distribution and behaviour of a tropical pelagic seabird: the wedge-tailed shearwater in the western Indian Ocean. Mar Ecol Prog Ser 391:231–242
Cherel Y, Bocher P, de Broyer P, Hobson KA (2002) Food and feeding ecology of the sympatric thin-billed Pachyptila belcheri and Antarctic P. desolata prions at Iles Kerguelen, Southern Indian Ocean. Mar Ecol Prog Ser 228:263–281
Cherel Y, Phillips RA, Hobson KA, McGill R (2006) Stable isotope evidence of diverse species-specific and individual wintering strategies in seabirds. Biol Lett 2:301–303
Cherel Y, Connan M, Jaeger A, Richard P (2014) Seabird year-round and historical feeding ecology: blood and feather δ13C and δ15N values document foraging plasticity of small sympatric petrels. Mar Ecol Prog Ser 505:267–280
Cherel Y, Quillfeldt P, Delord K, Weimerskirch H (2016) Combination of at-sea activity, geolocation and feather stable isotopes documents where and when seabirds moult. Front Ecol Evol 4:3
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Croxall JP, Prince PA (1980) Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biol J Linnean Soc 14:103–131
Dilley BJ, Davies D, Bond AL, Ryan PG (2015) Effects of mouse predation on burrowing petrel chicks at Gough Island. Antarct Sci 27:543–553
Fieberg J, Kochanny CO (2005) Quantifying home-range overlap: the importance of the utilization distribution. J Wildl Manag 69:1346–1359
Frere E, Quintana F, Gandini P, Wilson RP (2008) Foraging behaviour and habitat partitioning of two sympatric cormorants in Patagonia, Argentina. Ibis 150:558–564
Furness RW, Birkhead TR (1984) Seabird colony distributions suggest competition for food supplies during the breeding season. Nature 311:655–656
Grant PR (1994) Ecological character displacement. Science 266:746–747
Grecian WJ, Witt MJ, Attrill MJ, Bearhop S, Becker PH, Egevang C, Furness RW, Godley BJ, González-Solís J, Grémillet D, Kopp M, Lescroël A, Matthiopoulos J, Patrick SC, Peter HU, Phillips RA, Stenhouse IJ, Votier SC (2016a) Seabird diversity hotspot linked to ocean productivity in the Canary Current Large Marine Ecosystem. Biol Lett 12:20160024
Grecian WJ, Taylor GA, Loh G, McGill RAR, Miskelly CM, Phillips RA, Thompson DR, Furness RW (2016b) Contrasting migratory responses of two closely-related seabirds to long-term climate change. Mar Ecol Prog Ser 559:231–242
Grosbois V, Thompson PM (2005) North Atlantic climate variation influences survival in adult fulmars. Oikos 109:273–290
Hedd A, Montevecchi WA, Phillips RA, Fifield DA (2014) Seasonal sexual segregation by monomorphic sooty shearwaters Puffinus griseus reflects different reproductive roles during the pre-laying period. PLoS ONE 9:e85572
Hobson KA, Clark RG (1993) Turnover of δ13C in cellular and plasma fractions of blood: implications for non-destructive sampling in avian dietary studies. Auk 110:638–641
Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrom 24:3456–3460
Jones CWP (2018) Comparative ecology of Pachyptila species breeding sympatrically at Gough Island. MSc dissertation, University of Cape Town. https://hdl.handle.net/11427/29646. Accessed 18 Feb 2019
Klages NTW, Cooper J (1992) Bill morphology and diet of a filter-feeding seabird: the broad-billed prion Pachyptila vittata at South Atlantic Gough Island. J Zool 227:385–396
Lascelles BG, Taylor PR, Miller MG, Dias MP, Oppel S, Torres L, Hedd A, Le Corre M, Phillips RA, Shaffer SA, Weimerskirch H (2016) Applying global criteria to tracking data to define important areas for marine conservation. Divers Distrib 22:422–431
Lewis S, Sherratt TN, Hamer KC, Wanless S (2001) Evidence of intra-specific competition for food in a pelagic seabird. Nature 412:816–819
Llido J, Garçon V, Lutjiharms JRE, Sudre J (2005) Event-scale blooms drive enhanced primary productivity at the Subtropical Convergence. Geophys Res Lett 32:L5611
Marchant S, Higgins PJ (1990) Handbook of Australian, New Zealand and Antarctic birds. Ratites to Ducks, vol 1. Oxford University Press, Melbourne
Masello JF, Mundry R, Poisbleau M, Demongin L, Voigt CC, Wikelski M, Quillfeldt P (2010) Diving seabirds share foraging space and time within and among species. Ecosphere 1:19
Matthiopoulos J (2003) The use of space by animals as a function of accessibility and preference. Ecol Model 159:239–268
Navarro J, Forero MG, González-Solís J, Igual JM, Bécares J, Hobson KA (2009) Foraging segregation between two closely related shearwaters breeding in sympatry. Biol Lett 5:545–548
Navarro J, Votier SC, Aguzzi J, Chiesa JJ, Forero MG, Phillips RA (2013) Ecological segregation in space, time and trophic niche of sympatric planktivorous petrels. PLoS ONE 8:e62897
Navarro J, Cardador L, Brown R, Phillips RA (2015) Spatial distribution and ecological niches of non-breeding planktivorous petrels. Sci Rep 5:12164
Onley D, Scofield P (2007) Field guide to the albatrosses, petrels and shearwaters of the world. Christopher Helm, London
Pakhomov EA, McQuaid CD (1996) Distribution of surface zooplankton and seabirds across the Southern Ocean. Polar Biol 16:271–286
Pedersen EJ, Miller DL, Simpson GL, Ross N (2019) Hierarchical generalized additive models in ecology: an introduction with mgcv. PeerJ 7:e6876. https://doi.org/10.7717/peerj.6876
Phalan B, Phillips RA, Silk JR, Afanasyev V, Fukuda A, Fox J, Catry P, Higuchi H, Croxall JP (2007) Foraging behaviour of four albatross species by night and day. Mar Ecol Prog Ser 340:271–286
Phillips RA, Hamer KC (1999) Lipid reserves, fasting capability and the evolution of nestling obesity in procellariiform seabirds. Proc R Soc Lond [Biol] 266:1329–1334
Phillips RA, Xavier JC, Croxall JP (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Briggs DR (2004) Accuracy of geolocation estimates for flying seabirds. Mar Ecol Prog Ser 266:265–272
Phillips RA, Silk JR, Croxall JP, Afanasyev V (2006) Year-round distribution of white-chinned petrels from South Georgia: relationships with oceanography and fisheries. Biol Conserv 129:336–347
Phillips RA, Croxall JP, Silk JRD, Briggs DR (2007) Foraging ecology of albatrosses and petrels from South Georgia: two decades of insights from tracking technologies. Aquat Conserv Mar Freshw Ecosyst 17:S6–S21
Phillips RA, Bearhop S, McGill RAR, Dawson DA (2009) Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160:795–806
Quillfeldt P, McGill RAR, Masello JF, Weiss F, Strange IJ, Brickle P, Furness RW (2008) Stable isotope analysis reveals sexual and environmental variability and individual consistency in foraging of thin-billed prions. Mar Ecol Prog Ser 373:137–148
Quillfeldt P, Masello JF, Navarro J, Phillips RA (2013) Year-round spatial segregation of two small petrel species in the South Atlantic. J Biogeogr 40:430–441
Quillfeldt P, Cherel Y, Masello JF, Delord K, McGill RA, Furness RW, Moodley Y, Weimerskirch H (2015a) Half a world apart? Overlap in nonbreeding distributions of Atlantic and Indian Ocean thin-billed prions. PLoS ONE 10:e0125007
Quillfeldt P, Cherel Y, Delord K, Weimerkirch H (2015b) Cool, cold or colder? Spatial segregation of prions and blue petrels is explained by differences in preferred sea surface temperatures. Biol Lett 11:20141090
Ryan PG (2007) Field guide to the animals and plants of Tristan da Cunha and Gough Island. Pisces Publications, Newbury
Ryan PG, Bourgeois K, Dromzeé S, Dilley BJ (2014) The occurrence of two bill morphs of prions Pachyptila vittata on Gough Island. Polar Biol 37:727–735
Schroeder ID, Sydeman WJ, Sarkar N, Thompson SA, Bograd SJ, Schwing FB (2009) Winter pre-conditioning of seabird phenology in the California Current. Mar Ecol Prog Ser 393:211–223
Shirihai H (2007) A complete guide to Antarctic wildlife, 2nd edn. A&C Black, London
Taylor RS, Friesen VL (2017) The role of allochrony in speciation. Mol Ecol 26:3330–3342
Tranquilla LAM, Montevecchi WA, Hedd A, Fifield DA, Burke CM, Smith PA, Regular PM, Robertson GJ, Gaston AJ, Phillips RA (2013) Multiple-colony winter habitat use by murres Uria spp. in the Northwest Atlantic Ocean: implications for marine risk assessment. Mar Ecol Prog Ser 472:287–303
Warham J (1990) The petrels: their ecology and breeding systems. Academic Press, London
Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP (2000) Fast and fuel-efficient? Optimal use of wind by flying albatrosses. Proc R Soc Lond [Biol] 267:1869–1874
Weiss F, Furness RW, McGill RAR, Strange IJ, Masello JF, Quillfeldt P (2009) Trophic segregation of Falkland Islands seabirds: insights from stable isotope analysis. Polar Biol 32:1753–1763
Wessel P (2001) Global distribution of seamounts inferred from gridded Geosat/ERS 1 altimetry. J Geophys Res 106:19431–19441
Whitehead TO, Kato A, Ropert-Coudert Y, Ryan PG (2016) Habitat use and diving behaviour of macaroni Eudyptes chrysolophus and eastern rockhopper E. chrysocome filholi penguins during the critical pre-moult period. Mar Biol 163:19
Wilson RP (2010) Resource partitioning and niche hypervolume overlap in free-living pygoscelid penguins. Funct Ecol 24:646–657
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC, London
Acknowledgements
For assistance in the field, we thank Alex Bond, Jan Bradley, Delia Davies, Ben Dilley, Bruce Dyer, Derren Fox, David Kinchin-Smith, Werner Kuntz, Alexis Osborne, Michelle Risi, Chris Taylor and Emma Witcutt. For laboratory work, we thank Laurie Johnson for preparing feathers for isotopic analysis and Ian Newton from UCT’s Stable Isotope Unit for analysis of feather samples. Thank you to Steffen Oppel for assistance with analysing the representativeness of the tracking data. Logistical and financial support was provided by the South African Department of Environmental Affairs, through the South African National Antarctic Programme (SANAP), the National Research Foundation (South Africa) through the FitzPatrick Institute of African Ornithology (University of Cape Town), the Royal Society for the Protection of Birds (RSPB) and the Natural Environment Research Council (Ref. NE/R0001017/1). The Tristan da Cunha Government provided permission to work at Gough Island.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Science Animal Ethics Committee (SFAEC) of the University of Cape Town approved the research protocols for this study (protocol number 2014/V10/PR) as part of a larger project entitled, “Population dynamics and conservation of Southern Ocean albatrosses and petrels”. Field procedures, i.e., deployments of similarly sized devices on other prion species, have been approved by the Animal Ethics Committees of the British Antarctic Survey.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jones, C.W., Phillips, R.A., Grecian, W.J. et al. Ecological segregation of two superabundant, morphologically similar, sister seabird taxa breeding in sympatry. Mar Biol 167, 45 (2020). https://doi.org/10.1007/s00227-020-3645-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-3645-7