Abstract
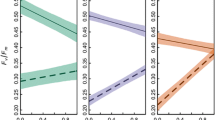
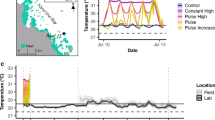
Coral reefs are diverse ecosystems relying on the presence of dinoflagellates (genus Symbiodinium), that are found in symbiotic association with multiple phyla and performing the majority of primary production. However, coral reefs are currently threatened by climate change and the increase in seawater temperature, which causes the bleaching phenomenon. While bleaching has been well documented for adult host organisms, it is still poorly understood in larval stages. We offered Symbiodinium types within clades A–F to the larvae of Mussismilia hispida (scleractinian coral), Berghia stephanieae (nudibranch) and Tridacna crocea (giant clam) and manipulated the temperature to 26, 29 or 32 °C. Samples were taken at 0, 12, 24, 48 and 72 h post-temperature increase, chlorophyll-a (chl-a) was extracted and its content measured in a fluorometer. Symbiodinium type, temperature and time all influenced chl-a content. M. hispida larvae displayed a bleaching threshold at 29 °C; larvae containing Symbiodinium A–F all bleached at 32 °C, but with significantly lower bleaching in larvae associated with type A1. B. stephanieae digested the symbionts; while chl-a content decreased over time equally for all clades, it is not possible to determine if it is related to bleaching. T. crocea larvae at 29 °C bleached for all symbiont types, except for A1. At 32 °C, all types were bleached, but type A1 bleached significantly less. These findings show that type A1 seems to be more thermo-tolerant in larvae of the tested species. This may be related to the fact that strains within this clade are homologous to both M. hispida and T. crocea, as they are found within these adult host’ tissues. Therefore, symbiont type may have an important role in invertebrate larvae development and present relevant implications for recruitment.




Similar content being viewed by others
References
Abrego D, van Oppen MJH, Willis BL (2009) Highly infectious symbiont dominates initial uptake in coral juveniles. Mol Ecol 18:3518–3531
Albright R, Mason B, Miller M, Langdon C (2010) Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc Natl Acad Sci USA 107:20400–20404
Allemand D, Furla P, Bénazet-Tambutté S (1998) Mechanisms of carbon acquisition for endosymbiont photosynthesis in Anthozoa. Can J Bot 76:925–941
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34:661–689
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Barneah O, Brickner I, Hooge M et al (2007) Three party symbiosis: acoelomorph worms, corals and unicellular algal symbionts in Eilat (Red Sea). Mar Biol 151:1215–1223
Belda-Baillie CA, Sison M, Silvestre V et al (1999) Evidence for changing symbiotic algae in juvenile tridacnids. J Exp Mar Biol Ecol 241:207–221
Brander LM, Van Beukering P, Cesar HSJ (2007) The recreational value of coral reefs: a meta-analysis. Ecol Econom 63:209–218
Brown BE (1997) Adaptations of reef corals to physical environmental stress. Adv Mar Biol 31:221–299
Carlos AA, Baillie BK, Maruyama T (2000) Diversity of dinoflagellate symbionts (zooxanthellae) in a host individual. Mar Ecol Prog Ser 195:93–100
Carroll DJ, Kempf SC (1990) Laboratory culture of the aeolid nudibranch Berghia verrucicornis (Mollusca, Opisthobranchia): some aspects of its development and life history. Biol Bull 179:243–253
Cesar HS (2000) Coral reefs: their functions, threats and economical value. In: Cesar HS (ed) Collected Essays on the Economics of Coral Reefs. Cordio, Kalmar, pp 14–39
Chen CA, Lam KK, Nakano Y, Tsai WS (2003) A stable association of the stress-tolerant zooxanthellae, Symbiodinium clade D, with the low-temperature-tolerant coral, Oulastrea crispata (Scleractinia: Faviidae) in subtropical non-reefal coral communities. Zool Stud 42:540–550
Colombo-Pallotta MF, Rodríguez-Román A, Iglesias-Prieto R (2010) Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29:899–907
Cooper TF, Ulstrup KE, Dandan SS et al (2011) Niche specialization of reef-building corals in the mesophotic zone: metabolic trade-offs between divergent Symbiodinium types. Proc R Soc B 278:1840–1850
Cumbo VR, Baird AH, van Oppen MJH (2012) The promiscuous larvae: flexibility in the establishment of symbiosis in corals. Coral Reefs 32:111–120
Cumbo VR, Fan TY, Edmunds PJ (2013) Effects of exposure duration on the response of Pocillopora damicornis larvae to elevated temperature and high pCO2. J Exp Mar Biol Ecol 439:100–107
De Lima LA, Migliolo L, Castro CBE et al (2013) Identification of a novel antimicrobial peptide from Brazilian coast coral Phyllogorgia dilatata. Protein Pept Lett 20:1153–1158
DeBoer TS, Baker AC, Erdmann MV et al (2012) Patterns of Symbiodinium distribution in three giant clam species across the biodiverse Bird’s Head region of Indonesia. Mar Ecol Prog Ser 444:117–132
Edmunds PJ, Gates RD, Leggat W et al (2005) The effect of temperature on the size and population density of dinoflagellates in larvae of the reef coral Porites astreoides. Invertebr Biol 124:185–193
Fitt WK, Spero HJ, Halas J et al (1993) Recovery of the coral Montastrea annularis in the Florida keys after the 1987 Caribbean “bleaching event”. Coral Reefs 12:57–64
Fitt WK, Brown BE, Warner ME, Dunne RP (2001) Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20:51–65
Furla P, Allemand D, Shick JM, Ferrier-Pagès C, Richier S, Plantivaux A, Merle PL, Tambutté S (2005) The symbiotic anthozoan: a physiological chimera between alga and animal. Integr Comp Biol 45:595–604
Glynn PW (1996) Coral reef bleaching: facts, hypotheses and implications. Global Change Biol 2:495–509
Grant AJ, Rémond M, People J, Hinde R (1997) Effects of host-tissue homogenate of the scleractinian coral Plesiastrea versipora on glycerol metabolism in isolated symbiotic dinoflagellates. Mar Biol 128:665–670
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms, I: Cycotella nana hustedl, and Detonula confervacea (cleve) gran. Can J Microbiol 8:229–239
Haryanti D, Yasuda N, Harii S, Hidaka M (2015) High tolerance of symbiotic larvae of Pocillopora damicornis to thermal stress. Zool Stud 54:52
Heslinga GA, Watson TC, Isamu T (1990) Giant clam farming. Pacific Fisheries Development Foundation (NMFS/NOAA), Honolulu
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshwater Res 50:839–866
Hoegh-Guldberg O, Mumby PJ, Hooten AJ et al (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hughes TP, Baird AH, Bellwood DR et al (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Hughes TP, Rodrigues MJ, Bellwood DR et al (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365
Iglesias-Prieto R, Matta JL, Robins WA, Trench RK (1992) Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc Natl Acad Sci USA 89:10302–10305
Jokiel PL, Rodgers KS, Kuffner IB et al (2008) Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27:473–483. https://doi.org/10.1007/s00338-008-0380-9
Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant, Cell Environ 21:1219–1230. https://doi.org/10.1046/j.1365-3040.1998.00345.x
Knowlton N, Brainard RE, Fisher R et al (2010) Coral reef biodiversity. In: McIntyre AD (ed) Life in the world’s oceans: diversity, distribution, and abundance. Blackwell Publishing, Oxford, pp 65–77
Ladner JT, Barshis DJ, Palumbi SR (2012) Protein evolution in two co-occurring types of Symbiodinium: an exploration into the genetic basis of thermal tolerance in Symbiodinium clade D. BMC Evol Biol 12:217
LaJeunesse TC, Trench RK (2000) Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol Bull 199:126–134
Leal MC, Nunes C, Alexandre D et al (2012) Parental diets determine the embryonic fatty acid profile of the tropical nudibranch Aeolidiella stephanieae: the effect of eating bleached anemones. Mar Biol 159:1745–1751
Leal MC, Ferrier-Pagès C, Petersen D, Osinga R (2017) Corals. Wiley-Blackwell Publishing, United Kingdom
Leggat W, Buck BH, Grice A, Yellowlees D (2003) The impact of bleaching on the metabolic contribution of dinoflagellate symbionts to their giant clam host. Plant, Cell Environ 26:1951–1961
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Little AF, van Oppen MJH, Willis BL (2004) Flexibility in algal endosymbioses shapes growth in reef corals. Science 304:1492–1494
Lohbeck KT, Riebesell U, Reusch TBH (2012) Adaptive evolution of a key phytoplankton species to ocean acidification. Nat Geosci 5:346–351. https://doi.org/10.1038/ngeo1441
McGinty ES, Pieczonka J, Mydlarz LD (2012) Variations in reactive oxygen release and antioxidant activity in multiple Symbiodinium types in response to elevated temperature. Microb Ecol 64:1000–1007
Mieog JC, Olsen JL, Berkelmans R et al (2009) The roles and interactions of symbiont, host and environment in defining coral fitness. PLoS ONE 4:e6364
Mies M, Sumida PYG (2012) Giant clam aquaculture: a review on induced spawning and larval rearing. Int J Mar Sci 2:62–69
Mies M, Braga F, Scozzafave MS et al (2012) Early development, survival and growth rates of the giant clam Tridacna crocea (Bivalvia: Tridacnidae). Braz J Oceanogr 60:129–135
Mies M, Chaves-Filho AB, Miyamoto S, Güth AZ, Tenório AA, Castro CB, Pires DO, Calderon EN, Sumida PYG (2017a) Production of three symbiosis-related fatty acids by Symbiodinium types in clades A-F associated with marine invertebrate larvae. Coral Reefs. https://doi.org/10.1007/s00338-017-1627-0
Mies M, Voolstra CR, Castro CB et al (2017b) Expression of a symbiosis-specific gene in Symbiodinium type A1 associated with coral, nudibranch and giant clam larvae. R Soc Open Sci 4:170253. https://doi.org/10.1098/rsos.170253
Moberg F, Folke C (1999) Ecological goods and services of coral reef ecosystems. Ecol Econom 29:215–233
Morse ANC, Iwao K, Baba M et al (1996) An ancient chemosensory mechanism new life to coral reefs. Biol Bull 191:149–154
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in coral reefs. In: Dubinsky Z (ed) Ecosystems of the World. Elsevier, Amsterdam, pp 75–87
Nesa B, Baird AH, Harii S et al (2012) Algal symbionts increase DNA damage in coral planulae exposed to sunlight. Zool Stud 51:12–17
Neves EG, Pires DO (2002) Sexual reproduction of Brazilian coral Mussismilia hispida (Verrill, 1902). Coral Reefs 21:161–168
Newton K, Côté IM, Pilling GM et al (2007) Current and future sustainability of island coral reef fisheries. Curr Biol 17:655–658
Olivotto I, Planas M, Simões N et al (2011) Advances in breeding and rearing marine ornamentals. J World Aquac Soc 42:135–166
Pandolfi JM, Bradbury RH, Sala E et al (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Picciani N, Seiblitz IGL, de Paiva PC et al (2016) Geographic patterns of Symbiodinium diversity associated with the coral Mussismilia hispida (Cnidaria, Scleractinia) correlate with major reef regions in the Southwestern Atlantic Ocean. Mar Biol. https://doi.org/10.1007/s00227-016-3010-z
Pochon X, Gates RD (2010) A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol Phylogenet Evol 56:492–497
Porter JW, Fitt WK, Spero HJ et al (1989) Bleaching in reef corals: Physiological and stable isotopic responses. Proc Natl Acad Sci USA 86:9342–9346. https://doi.org/10.1073/pnas.86.23.9342
Ralph PJ, Larkum AWD, Kühl M (2005) Temporal patterns in effective quantum yield of individual zooxanthellae expelled during bleaching. J Exp Mar Biol Ecol 316:17–28
Rowan R, Powers DA (1991) Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar Ecol Prog Ser 71:65–73
Schnitzler CE, Hollingsworth LL, Krupp DA, Weis VM (2012) Elevated temperature impairs onset of symbiosis and reduces survivorship in larvae of the Hawaiian coral, Fungia scutaria. Mar Biol 159:633–642
Sheppard CRC, Davy SK, Pilling GM (2009) The biology of coral reefs. Oxford University Press, Oxford
Stat M, Carter D, Hoegh-Guldberg O (2006) The evolutionary history of Symbiodinium and scleractinian hosts—symbiosis, diversity, and the effect of climate change. Perspect Plant Ecol Evol Syst 8:23–43
Strychar KB, Coates M, Sammarco PW et al (2005) Loss of Symbiodinium from bleached soft corals Sarcophyton ehrenbergi, Sinularia sp. and Xenia sp. J Exp Mar Biol Ecol 320:159–177
van Oppen MJH, Mahiny AJ, Done TJ (2005) Geographic distribution of zooxanthella types in three coral species on the great barrier reef sampled after the 2002 bleaching event. Coral Reefs 24:482–487
Venn AA, Wilson MA, Trapido-Rosenthal HG et al (2006) The impact of coral bleaching on the pigment profile of the symbiotic alga, Symbiodinium. Environment 29:2133–2142
Venn AA, Loram JE, Douglas AE (2008) Photosynthetic symbioses in animals. J Exp Bot 59:1069–1080
Vermeij MJA, Frade PR, Bak RPM (2013) Zooxanthellae presence acts as a settlement cue for aposymbiotic planulae of the caribbean coral Montastraea faveolata. Caribb J Sci 47:31–36
Warner WK, Fitt WK, Schmidt GW (1996) The effects of elevated temperature on the photosynthetic efficiency of zooxantheliae in hospite from four different species of reef coral: a novel approach. Plant, Cell Environ 19:291–299
Wasmund N, Topp I, Schories D (2006) Optimising the storage and extraction of chlorophyll samples. Oceanologia 48:125–144
Weis VM (2008) Cellular mechanisms of cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211:3059–3066
Weis VM, Reynolds WS, DeBoer MD, Krupp DA (2001) Host-symbiont specificity during onset of symbiosis between the dinoflagellates Symbiodinium spp. and planula larvae of the scleractinian coral Fungia scutaria. Coral Reefs 20:301–308
Welschmeyer NA (1994) Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol Oceanogr 39:1985–1992
Winter APM, Chaloub RM, Duarte GAS, Castro CB (2016) Photosynthetic responses of corals Mussismilia harttii (Verrill, 1867) from turbid waters to changes in temperature and presence/absence of light. Braz J Oceanogr 64:203–216
Yakovleva IM, Baird AH, Yamamoto HH et al (2009) Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar Ecol Prog Ser 378:105–112
Acknowledgements
We would like to thank Linda Waters, Stephen Kempf and William Fitt for kindly reviewing the manuscript and providing important notes. We also thank Mary Alice Coffroth and the Buffalo Undersea Reef Research Collection for supplying the Symbiodinium cultures, Flávia Saldanha-Corrêa for maintaining them, the Coral Vivo Institute staff, and Salvador Gaeta for the use of his facilities and equipment. This work was supported by Projeto Coral Vivo and sponsored by Petrobras and Arraial d’Ajuda Eco Parque. PYGS acknowledges Grants 302526/2012-9 and 2010/20350-8 from CNPq and FAPESP.
Author information
Authors and Affiliations
Contributions
MM designed the experiment, MM performed the experiment, CBC, DOP, ENC, MP and PYGS contributed with infrastructure/material/technical support, MM and AZG analyzed the data and MM and PYGS wrote the manuscript.
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors have no conflict of interest.
Additional information
Responsible Editor: R. Hill.
Reviewed by C. Zilberberg and an undisclosed expert.
Rights and permissions
About this article
Cite this article
Mies, M., Güth, A.Z., Castro, C.B. et al. Bleaching in reef invertebrate larvae associated with Symbiodinium strains within clades A–F. Mar Biol 165, 6 (2018). https://doi.org/10.1007/s00227-017-3263-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3263-1