Abstract
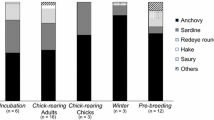
It is common for marine animals to be selective in the amount and quality of foods during reproduction due to the higher energetic demands. As central place foragers, seabirds are often selective with the prey species they deliver to their offspring. We evaluated trophic segregation between parents and their offspring in blue-footed booby (Sula nebouxii) by measuring stable isotope ratios (δ15N and δ13C) in blood samples during two breeding seasons and at different reproductive stages (incubation, early and late chick rearing). Additionally, we examined if δ15N values from chicks were correlated with their condition as reflected in blood alkaline phosphatase (ALP) levels. δ15N values increased and δ13C values decreased in adults as the breeding season progressed, indicating differences in foraging regions or the assimilated prey. δ15N values differed significantly between sexes; males had higher δ15N values than females during all reproductive stages sampled during the 2011 season, whereas in 2012 the difference between sexes was only observed during incubation. Offspring had higher δ15N values and lower δ13C values than adults, suggesting that, when feeding their chicks, parents feed them with prey from higher trophic levels and possibly from more oceanic environments. ALP levels, a proxy for bone growth and condition, measured in the offspring were positively correlated with δ15N values during the chick-rearing period. Although the diet of parents differed between reproductive stages, a multi-source Bayesian mixing model indicated that their primary prey were Pacific anchovy and halfbeaks, with a combined modal fractional contribution of 50–70% of the diet. The diet of the offspring was dominated by Pacific anchovy (≈36%), which was also the prey with the highest lipid content (C:N ratio 5.57), whereas the contributions of other fish species (or groups) were similar (17–24% each). Our findings suggest that parents actively feed their young with high-quality prey, which seems to increase some of the individual fitness components associated with better growth.



Similar content being viewed by others
References
Albano N, Masero JA, Sánchez-Guzmán JM, Villegas A, Santiago-Quesada F (2011) Effects of diet on growth-related patterns of energy and macronutrient assimilation efficiency in a semi-precocial bird, the Gull-billed Tern Gelochelidon nilotica. Ardea 99:93–101. doi:10.5253/078.099.0111
Alonso H, Granadeiro JP, Paiva VH, Dias AS, Ramos JA, Catry P (2012) Parent–offspring dietary segregation of Cory’s shearwaters breeding in contrasting environments. Mar Biol 159:1197–1207. doi:10.1007/s00227-012-1900-2
Ancona S, Sánchez-Colón S, Rodríguez C, Drummond H (2011) El Niño in the warm tropics: local sea temperature predicts breeding parameters and growth of blue-footed boobies. J Anim Ecol 80:799–808. doi:10.1111/j.1365-2656.2011.01821.x
Ancona S, Calixto-Albarrán I, Drummond H (2012) Effect of El Niño on the diet of a specialist seabird, Sula nebouxii, in the warm eastern tropical Pacific. Mar Ecol Prog Ser 462:273–286. doi:10.3354/meps09851
Anderson DJ, Ricklefs RE (1992) Brood size and food provisioning in masked and Blue-footed Boobies (Sula spp.). Ecology 73:1363–1374. doi:10.2307/1940682
Baird PH (1990) Influence of abiotic factors and prey distribution on diet and reproductive success of three seabird species in Alaska. Ornis Scand 21:224–235. doi:10.2307/3676782
Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012. doi:10.1111/j.0021-8790.2004.00861.x
Bond AL, McClelland GTW, Jones IL, Lavers JL, Kyser TK (2010) Stable isotopes confirm community patterns in foraging among Hawaiian Procellariiformes. Waterbirds 33:50–58. doi:10.1675/063.033.0106
Castillo-Guerrero JA, Mellink E (2011) Occasional inter-sex differences in diet and foraging behavior of the blue-footed booby: maximizing chick rearing in a variable environment? J Ornithol 152:269–277. doi:10.1007/s10336-010-0575-z
Castillo-Guerrero JA, González-Medina E, Fernández G (2014) Seabird colonies of the small islands of Bahía Santa María-La Reforma, Sinaloa, México. Waterbirds 37:439–445. doi:10.1675/063.037.0412
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287. doi:10.3354/meps329281
Cherel Y, Hobson KA, Hassani S (2005) Isotopic discrimination factors between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol Biochem Zool 78:106–115. doi:10.1086/425202
Cherel Y, Le Corre M, Jaquemet S, Ménard F, Richard P, Weimerskirch H (2008) Resource partitioning within a tropical seabird community: new information from stable isotopes. Mar Ecol Prog Ser 366:281–291. doi:10.3354/meps07587
Crawley MJ (2007) The R book. Wiley, Chichester
Cruz LL, McGill RAR, Goodman SJ, Hamer KC (2012) Stable isotope ratios of a tropical marine predator: confounding effects of nutritional status during growth. Mar Biol 159:873–880. doi:10.1007/s00227-011-1864-7
Davies WE, Hipfner JM, Hobson KA, Ydenberg RC (2009) Seabird seasonal trophodynamics: isotopic patterns in a community of Pacific alcids. Mar Ecol Prog Ser 382:211–219. doi:10.3354/meps07997
Deguchi T, Wada A, Watanuki Y, Osa Y (2010) Seasonal changes of the at-sea distribution and food provisioning in rhinoceros auklets. Ecol Res 25:123–137. doi:10.1007/s11284-009-0639-9
Drummond H, Osorno JL, Torres R, García-Chavelas C, Merchant Larios H (1991) Sexual size dimorphism and sibling competition: implications for avian sex ratios. Am Nat 138:623–641. doi:10.1086/285238
Engilis A, Oring LW, Carrera E, Nelson JW, Martinez-Lopez A (1998) Shorebird surveys in Ensenada Pabellones and Bahia Santa Maria, Sinaloa, Mexico: critical winter habitats for Pacific Flyway shorebirds. Wilson Bull 110:332–341
Forero MG, Hobson KA, Bortolotti GR, Donázar JA, Bertellotti M, Blanco G (2002) Food resources utilisation by the Magellanic penguin evaluated through stable-isotope analysis: segregation by sex and age and influence on offspring quality. Mar Ecol Prog Ser 234:289–299. doi:10.3354/meps234289
Forero MG, González-Solís J, Hobson KA, Doñazar JA, Bertellotti M, Blanco G, Bortolotti GR (2005) Stable isotopes reveal trophic segregation by sex and age in the Southern Giant Petrel in two different food webs. Mar Ecol Prog Ser 296:107–113. doi:10.3354/meps296107
González-Medina E, Castillo-Guerrero JA, Santiago-Quesada F, Villegas A, Masero JA, Sánchez-Guzmán JM, Fernández G (2015) Regulation of breeding expenditure in the blue-footed booby (Sula nebouxii): an experimental approach. Anim Behav 108:9–16. doi:10.1016/j.anbehav.2015.06.025
González-Medina E, Castillo-Guerrero JA, Santiago-Quesada F, Villegas A, Masero JA, Sánchez-Guzmán JM, Fernández G (2016) Variations in parental rearing expenditures trigger short-term physiological effects on offspring in a long-lived seabird. Ibis 158:305–314. doi:10.1111/ibi.12346
González-Solís J, Croxall JP, Wood AG (2000) Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos 90:390–398. doi:10.1034/j.1600-0706.2000.900220.x
Grémillet D, Pichegru L, Kuntz G, Woakes AG, Wilkinson S, Crawford RJM, Ryan PG (2008) A junk-food hypothesis for gannets feeding on fishery waste. P Roy Soc Lond B Biol 275:1149–1156. doi:10.1098/rspb.2007.1763
Guerra M, Drummond H (1995) Reversed sexual size dimorphism and parental care: minimal division of labour in the blue-footed booby. Behaviour 132:479–496. doi:10.1163/156853995X00162
Guglielmo CG, Cerasale DJ, Eldermire C (2005) A field validation of plasma metabolite profiling to assess refueling performance of migratory birds. Physiol Biochem Zool 78:116–125. doi:10.1086/425198
Harding AMA, Hobson KA, Wojciech W, Dmoch K, Karnovsky NJ, Van Pelt TI, Lifjeld JT (2008) Can stable isotope (δ13C and δ15N) measurements of little auk (Alle alle) adults and chicks be used to track changes in high-Arctic marine foodwebs? Polar Biol 31:725–733. doi:10.1007/s00300-008-0413-4
Hedd A, Montevecchi WA (2006) Diet and trophic position of Leach’s Storm-Petrel Oceanodroma leucorhoa during breeding and moult, inferred from stable isotope analysis of feathers. Mar Ecol Prog Ser 322:291–301. doi:10.3354/meps322291
Hipfner JM, McFarlane-Tranquilla L, Addison B, Hobson KA (2013) Trophic responses to the hatching of offspring in a central-place foraging seabird. J Ornithol 154:965–970. doi:10.1007/s10336-013-0962-3
Hobson KA (1993) Trophic relationships among high Arctic seabirds: insights from tissue-dependent stable-isotope models. Mar Ecol Prog Ser 95:7–18. doi:10.3354/meps095007
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. The Condor 94:189–197. doi:10.2307/1368808
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63:786–798
Hodum PJ, Hobson KA (2000) Trophic relationships among Antarctic fulmarine petrels: insights into dietary overlap and chick provisioning strategies inferred from stable- isotope (δ15N and δ13C) analyses. Mar Ecol Prog Ser 198:273–281. doi:10.3354/meps198273
Huato-Soberanis L, Lluch-Belda D (1987) Mesoscale cycles in the series of environmental indices related to the sardine fishery in the Gulf of California. Calif Coop Ocean Fish Invest Rep 28:128–134
Lluch-Belda D, Magallón BFJ, Schwartzlose RA (1986) Large fluctuations in the sardine fishery in the Gulf of California: possible causes. Calif Coop Ocean Fish Invest Rep 27:136–140
Maness TJ, Anderson DJ (2013) Predictors of juvenile survival in birds. Ornithol Monogr 78:1–55. doi:10.1525/om.2013.78.1.1
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, play later? Trends Ecol Evol 16:254–260. doi:10.1016/S0169-5347(01)02124-3
Navarro J, González-Solís J (2009) Environmental determinants of foraging strategies in Cory’s shearwaters Calonectris diomedea. Mar Ecol Progr Ser 378:259–267. doi:10.3354/meps07880
Nelson JB (1978) The Sulidae: gannets and boobies, 1st edn. Oxford University Press, Oxford
Nelson JB (2005) Pelicans, cormorants and their relatives, the Pelecaniforms, 1st edn. Oxford University Press, Oxford
Parnell A, Inger R, Bearhop S, Jackson AL (2008) Stable isotope analysis in R (SIAR). http://cran.r-project.org/web/packages/siar/index.html. Accessed 28 Sept 2015
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS One 5:e9672. doi:10.1371/journal.pone.0009672
Post DM, Layman CA, Albrey Arrington D, TakimotoG, Quattochi J, Montana CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. doi:10.1007/s00442-006-0630-x. Accessed 28 Sept 2015
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org
Raya Rey A, Polito M, Archuby D, Coria N (2012) Stable isotopes identify age- and sex-specific dietary partitioning and foraging habitat segregation in Southern Giant Petrels breeding in Antarctica and Southern Patagonia. Mar Biol 159:1317–1326. doi:10.1007/s00227-012-1912-y
Rector ME, Kouwenberg AL, Wilhelm SI, Robertson GJ, McKay DW, Fitzsimmons MG, Baker CR, Cameron-MacMillan ML, Walsh CJ, Storey AE (2012) Corticosterone levels of Atlantic puffins vary with breeding stage and sex but are not elevated in poor foraging years. Gen Comp Endocr 178:408–416. doi:10.1016/j.ygcen.2012.06.008
Romano MD, Piatt JF, Roby DD (2006) Testing the junk-food hypothesis on marine birds: effect of prey type on growth and development. Waterbirds 29:407–414. doi:10.1675/1524-4695(2006)29[407:TTJHOM]2.0.CO;2
Sears J, Hatch SA, O’Brien DM (2009) Disentangling effects of growth and nutritional status on seabirds stable isotope ratios. Oecologia 159:41–48. doi:10.1007/s00442-008-1199-3
Shaffer SA, Costa DP, Weimerskirch H (2003) Foraging effort in relation to the constraints of reproduction in free-ranging albatrosses. Funct Ecol 17:66–74. doi:10.1046/j.1365-2435.2003.00705.x
Shealer DA (2002) Foraging behaviour and food of seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds, 1st edn. CRC Press, Florida, pp 179–216
Thierry AM, Ropert-Coudert Y, Raclot T (2013) Elevated corticosterone levels decrease reproductive output of chick-rearing Adélie penguins but do not affect chick mass at fledging. Conserv Physiol 1:1–12. doi:10.1093/conphys/cot007
Tilgar V, Mänd R, Ots I, Mägi M, KilgasP, Reynolds SJ (2004a) Calcium availability affects bone growth in nestlings of free-living great tits (Parus major), as detected by plasma alkaline phosphatase. J Zool Lond 263:269–274. doi:10.1017/S0952836904005254
Tilgar V, Ots I, Mänd R (2004b) Bone alkaline phosphatase as a sensitive indicator of skeletal development in birds: a study of great tit nestlings. Physiol Biochem Zool 77:530–535. doi:10.1086/420947
Tilgar V, Kilgas P, Viitak A, Reynolds SJ (2008) The rate of bone mineralization in birds is directly related to alkaline phosphatase activity. Physiol Biochem Zool 81:106–111. doi:10.1086/523305
Torres R, Drummond H (1999) Does large size make daughters of the blue-footed booby more expensive than sons? J Anim Ecol 68:1133–1141. doi:10.1046/j.1365-2656.1999.00357.x
Villegas A, Sánchez JM, Costillo E, Corbacho C (2002) Blood chemistry and haematocrit of the black vulture (Aegypius monachus). Comp Biochem Physiol A 132:489–497. doi:10.1016/S1095-6433(02)00097-1
Villegas A, Masero JA, Corbacho C, Gutiérrez JS, Albano N, Sánchez-Guzmán JM (2013) Sex-specific vulnerability to breeding conditions in chicks of the sexually monomorphic Gull-billed Tern. J Ornithol 154:431–439. doi:10.1007/s10336-012-0907-2
Viñuela J, Ferrer M (1997) Regulation of growth in red kites and imperial eagles. Wilson Bull 109:92–101
Weimerskirch H, Shaffer SA, Tremblay Y, Costa DP, Gadenne H, Kato A, Ropert-Coudert Y, Sato K, Aurioles D (2009) Species- and sex-specific differences in foraging behaviour and foraging zones in blue-footed and brown boobies in the Gulf of California. Mar Ecol Prog Ser 391:267–278. doi:10.3354/meps07981
Williams CT, Buck CL, Sears J, Kitaysky AS (2007) Effects of nutritional restriction on nitrogen and carbon stable isotopes in growing seabirds. Oecologia 153:11–18. doi:10.1007/s00442-007-0717-z
Williams CT, Iverson SJ, Buck CL (2008) Stable isotopes and fatty acid signatures reveal age- and stage-dependent foraging niches in tufted puffins. Mar Ecol Prog Ser 363:287–298. doi:10.3354/meps07477
Zavalaga CB, Benvenutti S, Dall’Antonia L, Emslie SD (2007) Diving behavior of the blue-footed boobies Sula nebouxii in northern Peru in relation to sex, body size and prey type. Mar Ecol Prog Ser 336:291–303. doi:10.3354/meps336291
Zavalaga CB, Benvenuti S, Dall’Antonia L, Emslie SD (2008) Foraging areas of breeding blue-footed boobies Sula nebouxii in northern Peru, as determined by direction recorders. J Avian Biol 39:405–412. doi:10.1111/j.0908-8857.2008.04275.x
Acknowledgements
We thank M. Guevara, A. Mendoza, M. Leal, M. Arvizú, S. Rendón, M. Lerma, F. Quesada, J.P. Ceyca, A. Leal, D. Brito, C. Franco, and N. Albano for their help during fieldwork; M.R. Barradas for laboratory support. We thank Ann Grant and the two anonymous reviewers for providing thoughtful recommendations that improved the manuscript. EGM was supported by a PhD student scholarship provided by CONACYT (Programa de Doctorado en Ciencias del Mar y Limnología, UNAM #201218).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Fondo Mexicano para la Conservación de la Naturaleza A.C. (PIE-2012-A-P-C-IGSI-12-12), CONACYT (No. I010/176/2012), Sonoran Joint Venture and Pronatura México A.C.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. Sample and data collections complied with current Mexican laws and were carried out under permits from the Dirección General de Vida Silvestre (SGPA/DGVS/08559/11). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Communicated by Y. Cherel.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
González-Medina, E., Castillo-Guerrero, J.A., Herzka, S.Z. et al. Flexibility in food resource allocation in parents and selectivity for offspring: variations in δ15N and δ13C values during breeding of the blue-footed booby. Mar Biol 164, 38 (2017). https://doi.org/10.1007/s00227-017-3070-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3070-8