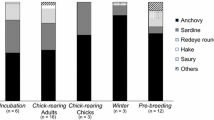
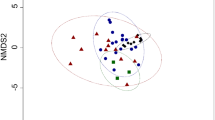
Abstract
The foraging challenge for predators is to find and capture food with adequate levels of energy and nutrients. Marine predators require particularly sophisticated foraging strategies that enable them to balance self- and offspring-feeding, and also in many circumstances simultaneously consider the nutritional constraints of their partners. Here we combined the use of dietary analysis, proximate composition and nutritional geometry (right-angled mixture triangle nutritional models) to examine the macronutrient preferences of Australasian gannets (Morus serrator) at Farewell Spit gannetry in New Zealand. Our results showed intra- and inter-specific variation in the protein, lipid and water composition of prey captured by our sample of 111 Australasian gannets. In addition, we observed significant differences in the Australasian gannets’ nutritional niche between seasons. We provide evidence of sex-specific macronutrient foraging strategies in a successful marine predator in the wild. We have shown that in spite of fluctuations in the nutritional composition of foods available to Australasian gannets, males consistently capture prey with higher protein-to-lipid ratios and lower lipid-to-water ratios than females. These results aid to better understand the evolutionary relationship between macronutrient selection and sex-specific traits in wild animals. They also suggest an incentive for these predators to combine individually imbalanced but nutritionally complementary foods to achieve dietary balance, further highlighting the likelihood that prey selection is guided by the balance of macronutrients, rather than energy alone.




Similar content being viewed by others
Notes
http://doi.pangaea.de/10.1594/PANGAEA.858335
References
Angel LP, Wells MR, Rodríguez-Malagón MA, Tew E, Speakman JR, Arnould JPY (2015) Sexual size dimorphism and body condition in the Australasian Gannet. PLoS One 10:e0142653
Barbraud C (2000) Natural selection on body size traits in a long-lived bird, the snow petrel Pagodroma nivea. J Evol Biol 13:81–88
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Ecol Prog Ser 311:157–164
Bijleveld AI, Mullers RH (2009) Reproductive effort in biparental care: an experimental study in long-lived Cape gannets. Behav Ecol 20:736–744
Chiaradia A, Forero MG, Hobson KA, Cullen JM (2010) Changes in diet and trophic position of a top predator 10 years after a mass mortality of a key prey. ICES J Mar Sci 67:1710–1720
Cleasby IR, Wakefield ED, Bodey TW, Davies RD, Patrick SC, Newton J, Votier SC, Bearhop S, Hamer KC (2015) Sexual segregation in a wide-ranging marine predator is a consequence of habitat selection. Mar Ecol Prog Ser 518:1–12
Duffy DC, Jackson S (1986) Diet studies of seabirds: a review of methods. Colon Waterbirds 9:1–17
Duke GE (1997) Gastrointestinal physiology and nutrition in wild birds. Proc Nut Soc 56:1049–1056
Elliott KH, Gaston AJ, Crump D (2010) Sex-specific behavior by a monomorphic seabird represents risk partitioning. Behav Ecol 21:1024–1032
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Fryxell JM, Lundberg P (1997) Individual behavior and community dynamics. Chapman and Hall, New York
Galef BG (1996) Food selection: problems in understanding how we choose foods to eat. Neurosci Biobehav Rev 20:67–73
González-Solís J, Croxall JP, Wood AG (2000) Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos 90:390–398
Gray CM, Hamer KC (2001) Food-provisioning behaviour of male and female Manx shearwaters, Puffinus puffinus. Anim Behav 62:117–121
Grémillet D, Pichegru L, Kuntz G, Woakes AG, Wilkinson S, Crawford RJM, Ryan PG (2008) A junk-food hypothesis for gannets feeding on fishery waste. Proc R Soc B 275:1149–1156
Hailey A, Chidavaenzi RL, Loveridge JP (1998) Diet mixing in the omnivorous tortoise Kinixys spekii. Funct Ecol 12:373–385
Hamer KC, Phillips RA, Wanless S, Harris MP, Wood AG (2000) Foraging ranges, diets and feeding locations of gannets (Morus bassanus) in the North Sea: evidence from satellite telemetry. Mar Ecol Prog Ser 200:257–264
Hamer KC, Quillfeldt P, Masello JF, Fletcher KL (2006) Sex differences in provisioning rules: responses of Manx shearwaters to supplementary chick feeding. Behav Ecol 17:132–137
Handley S, Sagar P, Schuckard R (2011) Seabird, marine mammal and surface fish surveys of Tasman and Golden Bay, Nelson. Part B: Stable Isotopes of seabirds and prey-fish. NIWA Report
Hedd A, Montevecchi WA, Phillips RA, Fifield DA (2014) Seasonal sexual segregation by monomorphic sooty shearwaters Puffinus griseus reflects different reproductive roles during the pre-laying period. PLoS One 9:e85572
Hilton GM, Furness RW, Houston DC (2000) The effects of diet switching and mixing on digestion in seabirds. Funct Ecol 14:145–154
Hobday AJ, Young JW, Abe O, Costa DP, Cowen RK, Evans K, Gasalla MA, Kloser R, Maury O, Weng KC (2013) Climate impacts and oceanic top predators: moving from impacts to adaptation in oceanic systems. Rev Fish Biol Fish 23:537–546
Ismar SMH (2010) Foraging and breeding ecology of the Australasian gannet (Morus serrator), with applications for rare New Zealand seabirds. Ph.D. Thesis, The University of Auckland, New Zealand
Jensen K, Mayntz D, Toft S, Raubenheimer D, Simpson SJ (2011) Prey nutrient composition has different effects on Pardosa wolf spiders with dissimilar life histories. Oecologia 165:577–583
Jensen K, Mayntz D, Toft S, Clissold FJ, Hunt J, Raubenheimer D, Simpson SJ (2012) Optimal foraging for specific nutrients in predatory beetles. Proc R Soc B 279:2212–2218
Kronmal RA (1993) Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc A 156:379–392
Kuznetsova A, Brockhoff PB, Christensen RHB (2015) lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package version 2.0-25. http://CRAN.R-project.org/package=lmerTest
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lenky C, Eisert R, Oftedal OT, Metcalf V (2012) Proximate composition and energy density of nototheniid and myctophid fish in McMurdo Sound and the Ross Sea, Antarctica. Polar Biol 35:717–724
Lewis S, Benvenuti S, Dall’Antonia L, Griffiths RG, Money L, Sherratt TN, Wanless S, Hamer KC (2002) Sex-specific foraging behaviour in a monomorphic seabird. Proc R Soc B 269:1687–1693
Lewis S, Hamer KC, Money L, Griffiths R, Wanless S, Sherratt TN (2004) Brood neglect and contingent foraging behaviour in a pelagic seabird. Behav Ecol Sociobiol 56:81–88
Machovsky-Capuska GE, Dwyer SL, Alley MR, Stockin KA, Raubenheimer D (2011a) Evidence for fatal collisions and kleptoparasitism while plunge diving in gannets. Ibis 153:631–635
Machovsky-Capuska GE, Huynen L, Lambert D, Raubenheimer D (2011b) UVS is rare in seabirds. Vis Res 51:1333–1337
Machovsky-Capuska GE, Howland HC, Vaughn RL, Würsig B, Raubenheimer D, Hauber ME, Katzir G (2012) Visual accommodation and active pursuit of prey underwater in a plunge diving bird: the Australasian gannet. Proc R Soc B 279:4118–4125
Machovsky-Capuska GE, Hauber ME, Dassis M, Libby E, Wikelski MC, Schuckard R, Melville D, Cook W, Houston M, Raubenheimer D (2014a) Foraging behaviour and habitat use of chick-rearing Australasian Gannets in New Zealand. J Ornithol 155:379–387
Machovsky-Capuska GE, Hauber ME, Libby E, Amiot C, Raubenheimer D (2014b) The contribution of private and public information in foraging by Australasian gannets. Anim Cogn 17:849–858
Machovsky-Capuska GE, Senior AM, Simpson SJ, Raubenheimer D (2016) The multi-dimensional nutritional niche. Trends Ecol Evol doi:10.1016/j.tree.2016.02.009
Madigan DJ, Carlisle AB, Dewar H, Snodgrass OE, Litvin SY, Micheli F, Block BA (2012) Stable isotope analysis challenges wasp-waist food web assumptions in an upwelling pelagic ecosystem. Sci Rep 2:654
Magalhães MC, Santos RS, Hamer KC (2008) Dual-foraging of Cory’s shearwaters in the Azores: feeding locations, behaviour at sea and implications for food provisioning of chicks. Mar Ecol Prog Ser 359:283–293
Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessman J, Clissold FJ, Zajitschek F, Lailvaux SP, Raubenheimer D, Bonduriansky R, Brooks RC (2008) Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr Biol 18:1062–1066
Malinowski CR, Herzing DL (2015) Prey use and nutritional differences between reproductive states and age classes in Atlantic spotted dolphins (Stenella frontalis) in the Bahamas. Mar Mam Sci 31:1471–1493
Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ (2005) Nutrient-specific foraging in invertebrate predators. Science 307:111–113
Mayntz D, Nielsen VH, Sørensen A, Toft S, Raubenheimer D, Hejlesen C, Simpson SJ (2009) Balancing of protein and lipid intake by a mammalian carnivore, the mink, Mustela vison. Anim Behav 77:349–355
Meynier L, Stockin KA, Bando MKH, Duignan PJ (2008) Stomach contents of common dolphin (Delphinus sp.) from New Zealand waters. N Z J Mar Freshw Res 42:257–268
Mock DW, Fujioka M (1990) Monogamy and long-term pair bonding in vertebrates. TREE 5:39–43
Montevecchi WA, Piatt JF (1984) Composition and energy contents of mature inshore spawning capelin (Mallotus villosus): implications for seabird predators. Comp Biochem Physiol A 78:15–20
Montevecchi WA, Piatt JF (1987) Dehydration of seabird prey during transport to the colony: effects on wet weight energy densities. Can J Zool 65:2822–2824
Montevecchi WA, Porter JM (1980) Parental investments by seabirds at the breeding area with emphasis on Northern Gannets, Morus bassanus. In: Burger J, Olla B, Winn H (eds) Behavior of marine animals: current perspectives in research. Marine birds, vol 4. Plenum Press, New York, pp 323–360
Montevecchi WA, Ricklefs RE, Kirkham IR, Gabaldon D (1984) Growth energetics of nestling northern gannets (Sula bassanus). Auk 101:334–341
Morehouse NI, Nakazawa T, Booher CM, Jeyasingh PD, Hall MD (2010) Sex in a material world: why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 119:766–778
Mullers RHE, Navarro RA (2010) Foraging behaviour of Cape gannets as an indicator of colony health status. Endang Species Res 12:193–202
Navarro RA (1992) Body composition, fat reserves, and fasting capability of cape gannet chicks. Wilson Bull 104:644–655
Nelson JB (1978) The sulidae: gannets and boobies. Oxford University Press, Oxford
Norman FI (2000) Preliminary investigation of the bycatch of marine birds and mammals in inshore commercial fisheries, Victoria, Australia. Biol Conserv 92:217–226
Österblom H, Olsson O, Blenckner T, Furness RW (2008) Junk-food in marine ecosystems. Oikos 117:967–977
Paiva VH, Geraldes P, Marques V, Rodríguez R, Garthe S, Ramos JA (2013) Effects of environmental variability on different trophic levels of the North Atlantic food web. Mar Ecol Prog Ser 477:15–28
Paulin C, Stewart A, Roberts C, McMillan P (1989) New Zealand Fish: a complete guide. National Museum of New Zealand Miscellaneous series no. 19
Peck DR, Congdon BC (2006) Sex-specific chick provisioning and diving behaviour in the wedge-tailed shearwater Puffinus pacificus. J Avian Biol 37:245–251
Phillips RA, Hamer KC (1999) Lipid reserves, fasting capability and the evolution of nestling obesity in procellariiform seabirds. Proc R Soc B 266:1329–1334
Pinet P, Jaquemet S, Phillips RA, Le Corre M (2012) Sex specific foraging strategies throughout the breeding season in a tropical, sexually monomorphic small petrel. Anim Behav 83:979–989
Quillfeldt P, Masello JF, Lubjuhn T (2006) Variation in the adult body mass of Wilson’s storm petrels Oceanites oceanicus during breeding. Polar Biol 29:372–378
Raubenheimer D (2011) Toward a quantitative nutritional ecology: the right-angled mixture triangle. Ecol Monogr 81:407–427
Raubenheimer D, Rothman JM (2013) Nutritional ecology of entomophagy in humans and other primates. Ann Rev Entomol 58:141–160
Raubenheimer D, Simpson SJ (1997) Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev 10:151–179
Raubenheimer D, Mayntz D, Simpson SJ, Tøft S (2007) Nutrient-specific compensation following diapause in a predator: implications for intraguild predation. Ecology 88:2598–2608
Raubenheimer D, Simpson SJ, Mayntz D (2009) Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct Ecol 23:4–16
Raubenheimer D, Simpson SJ, Tait AH (2012) Match and mismatch: conservation physiology, nutritional ecology and the timescales of biological adaptation. Philos Trans R Soc B 367:1628–1646
Raubenheimer D, Machovsky-Capuska GE, Chapman CA, Rothman JM (2015) Geometry of nutrition in field studies: an illustration using wild primates. Oecologia 177:223–234
R Core Team (2015) R: A language and environment for statistical computing. Version 3.2.1. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ricklefs RE (1990) Seabird life histories and the marine environment: some speculations. Colon Waterbirds 13:1–6
Ropert-Coudert Y, Grémillet D, Kato A, Ryan P, Naito Y, Le Maho Y (2004) A fine-scale time budget of Cape gannets provides insights into the foraging strategies of coastal seabirds. Anim Behav 67:985–992
Rothman JM, Chapman CA, Struhsaker TT, Raubenheimer D, Twinomugisha D, Waterman PG (2015) Long-term declines in nutritional quality of tropical leaves. Ecology 96:873–878
Schuckard R, Melville DS, Cook W, Machovsky-Capuska GE (2012) Diet of the Australasian gannet (Morus serrator) at Farewell Spit, New Zealand. Notornis 59:66–70
Senior AM, Nakagawa S, Lihoreau M, Simpson SJ, Raubenheimer D (2015) An overlooked consequence of dietary mixing: a varied diet reduces interindividual variance in fitness. Am Nat 186:649–659
Shaffer SA, Weimerskirch H, Costa DP (2001) Functional significance of sexual dimorphism in wandering albatrosses, Diomedea exulans. Funct Ecol 15:203–210
Simpson SJ, Raubenheimer D (2012) The nature of nutrition: an integrative framework from animal adaptation to human obesity. Princeton University Press, Princeton
Spitz J, Mourocq E, Schoen V, Ridoux V (2010) Proximate composition and energy content of forage species from the Bay of Biscay: high-or low-quality food? ICES J Mar Sci 67:909–915
Srinivasan M, Dassis M, Benn E, Stockin KA, Martinez E, Machovsky-Capuska GE (2015) Using non-systematic surveys to investigate regional climate change effects on Australasian gannets in the Hauraki Gulf, New Zealand. J Sea Res 99:74–82
Stahl JC, Sagar PM (2006) Long and short trips in nonbreeding Buller’s albatrosses: relationships with colony attendance and body mass. Condor 108:348–365
Stauss C, Bearhop S, Bodey TW, Garthe S, Gunn C, Grecian WJ, Inger R, Knight ME, Newton J, Patrick SC, Phillips RA, Waggitt JJ, Votier SC (2012) Sex-specific foraging behavior in northern gannets Morus bassanus: incidence and implications. Mar Ecol Prog Ser 457:151–162
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Symonds MR, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Tait A, Raubenheimer D, Stockin KA, Merriman M, Machovsky-Capuska GE (2014) Nutritional geometry of gannets and the challenges in field studies. Mar Biol 12:2791–2801
Tarnopolsky MA, Saris WH (2001) Evaluation of gender differences in physiology: an introduction. Curr Opin Clin Nutr Metab Care 4:489–492
Thaxter CB, Daunt F, Hamer KC, Watanuki Y, Harris YP, Grémillet D, Peters G, Wanless S (2009) Sex-specific food provisioning in a monomorphic seabird, the common guillemot Uria algae: nest defence, foraging efficiency or parental effort? J Avian Biol 40:75–84
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Wanless S, Harris MP, Redman P, Speakman JR (2005) Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea. Mar Ecol Prog Ser 294:1–8
Warton DI, Hui FKC (2010) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Weimerskirch H, Chastel O, Chaurand T, Ackerman L, Hindermeyer X, Judas J (1994) Alternate long and short foraging trips in pelagic seabird parent. Anim Behav 47:472–476
Weimerskirch H, Cherel Y, Cuenot-Chaillet F, Ridoux V (1997) Alternative foraging strategies and resource allocation by male and female wandering albatrosses. Ecology 78:2051–2063
Welcker J, Steen H, Harding A, Gabrielsen GW (2009) Sex-specific provisioning behaviour in a monomorphic seabird with a bimodal foraging strategy. Ibis 151:502–513
Westoby M (1978) What are the biological bases of varied diets? Am Nat 112:627–631
Wilder SM, Eubanks MD (2010) Might nitrogen limitation promote omnivory among carnivorous arthropods: comment. Ecology 91:3114–3117
Wright J, Cuthill I (1989) Manipulation of sex differences in parental care. Behav Ecol Sociobiol 25:171–181
Zavalaga CB, Benvenuti S, Dall’Antonia L, Emslie SD (2007) Diving behavior of blue-footed boobies Sula nebouxii in northern Peru in relation to sex, body size and prey type. Mar Ecol Prog Ser 336:291–303
Acknowledgments
We thank J. Melville, S. Dwyer, D. Boulton, P. Jones and S. Clements for their assistance during the sample collection. Special thanks to A. Teixeira-Pinto for assistance in early stages of the statistical analysis and C. Lea (Massey University) for assisting on the organization of the logistics for the analysis of the samples. We also thank to the anonymous referees and V. H. Paiva for useful comments that have enhanced the manuscript. Aspects of this work were funded by Faculty of Veterinary Science DVC compact fund (The University of Sydney). The Department of Conservation, Golden Bay (New Zealand) kindly allowed use of their house at Farewell Spit, and transport was provided by Paddy Gillooly of Farewell Spit Ecotours (New Zealand). This study was conducted under permits from the University of Sydney Animal Ethics committee (N00/7-2013/3/6016), Massey University Animal Ethics committee (13/65) and the New Zealand Department of Conservation (35189-FAU). D. R. is an Adjunct Professor in the New Zealand Institute for Advanced Study, Massey University.
Author contributions
G. E. M.-C., E. B., W. C., D. M., R. S., K. B., C. P. and M. O. collected the data. A. M. S., G. E. M.-C., E. B., C. P., A. H. T., W. C., B. W., K. B. and D. R. analysed the data. G. E. M.-C., A. M. S., E. B., A. H. T., D. M., C. P., M. O., B. W., K. A. S. and D. R., wrote and edited the manuscript. G. E. M.-C., D. R. and K. A. S. designed the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: V. Paiva.
Reviewed by L. Krüger and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Machovsky-Capuska, G.E., Senior, A.M., Benn, E.C. et al. Sex-specific macronutrient foraging strategies in a highly successful marine predator: the Australasian gannet. Mar Biol 163, 75 (2016). https://doi.org/10.1007/s00227-016-2841-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2841-y