Abstract
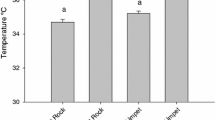
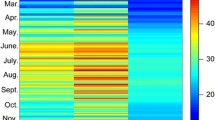
The rocky intertidal zone is among one of the most highly variable environments on Earth, with rapid and unpredictable changes in temperature on a daily basis. Numerous studies have investigated the thermal physiology of intertidal animals; however, few have focused on an organism’s capacity to withstand repeated heat stress during the emersion of low-tide periods and how previous exposures to sublethal increases in temperature may modulate the capacity to withstand severe heat stress. Lottia digitalis, a species of limpet ubiquitous along the Pacific coast of North America, were acclimated under ambient ocean conditions in the laboratory during the summer and winter months. Limpets were aerially exposed with or without preliminary heat exposures of varying magnitudes (15–35 °C for 2 h) the day before being challenged to a lethal temperature increase to investigate how sequential low-tide-period conditions affect upper thermal tolerance and temperature sensitivity. Limpets with a preliminary aerial exposure of 25–35 °C (summer) and 20 °C (winter) had greater upper critical thermal limits of cardiac performance as determined by final breakpoint temperature (~1.5–6 °C increase) and flatline temperature (~1–2 °C increase) than limpets with no previous exposure. The magnitude of temperature increase that conferred significant increases in thermal tolerance differed in summer and winter, reflecting seasonal differences in the thermal environment in nature. Fingered limpets’ upper thermal tolerance is plastic and likely modulated by the previous day’s low-tide exposure, demonstrating the importance of incorporating the repeated nature of stress into thermal physiology research in intertidal organisms.






Similar content being viewed by others
References
Anestis A, Pörtner HO, Michaelidis B (2010) Anaerobic metabolic patterns related to stress response in hypoxia exposed mussels Mytilus galloprovincialis. J Exp Mar Biol Ecol 394:123–133
Angilletta MJ, Zelic MH, Adrian GJ, Hurliman AM, Smith CD (2013) Heat tolerance during embryonic development has not diverged among populations of a widespread species (Sceloporus undulatus). Cons Physiol 1:1–9
Bergmeyer HU (1983) Methods of enzymatic analysis. Academic Press, New York
Bjelde BE, Todgham AE (2013) Thermal physiology of the fingered limpet Lottia digitalis under emersion and immersion. J Exp Biol 216:2858–2869
Blackmore DT (1969) Studies of Patella vulgata L. II. Seasonal variation in biochemical composition. J Exp Mar Biol Ecol 3:231–245
Branch GM (1981) The biology of limpets: physical factors, energy flow, and ecological interactions. Oceanogr Mar Biol 19:235–380
Chelazzi G, De Pirro M, Williams GA (2001) Cardiac responses to abiotic factors in two tropical limpets, occurring at different levels of the shore. Mar Biol 139:1079–1085
Chelazzi G, De Pirro M, Williams GA (2004) Different cardiac response to copper in limpets from metal polluted and clean shores of Hong Kong. Mar Environ Res 58:83–93
Chen X, Stillman JH (2012) Multigenerational analysis of temperature and salinity variability affects on metabolic rate, generation time, and acute thermal and salinity tolerance in Daphnia pulex. J Therm Biol 37:185–194
Connor KM, Gracey AY (2012) High-resolution analysis of metabolic cycles in the intertidal mussel Mytilus californianus. Am J Physiol Regul Integr Comp Physiol 302:R103–R111
Crummett LT, Eernisse DJ (2007) Genetic evidence for the cryptic species pair Lottia digitalis and Lottia austrodigitalis and microhabitat partitioning in sympatry. Mar Biol 152:1–13
Dahlgaard J, Loeschcke V, Michalak P, Justesen J (1998) Induced thermotolerance and associated expression of the heat-shock protein Hsp70 in adult Drosophila melanogaster. Funct Ecol 12:786–793
De Pirro M, Santini G, Chelazzi G (1999) Cardiac responses to salinity variations in two differently zoned Mediterranean limpets. J Comp Physiol B 169:501–506
De Pirro M, Chelazzi G, Borghini F, Focardi S (2001) Variations in cardiac activity following acute exposure to copper in three co-occurring but differently zoned Mediterranean limpets. Mar Pollut Bull 42:1390–1396
Denny MW, Harley CDG (2006) Hot limpets: predicting body temperature in a conductance-mediated thermal system. J Exp Biol 209:2409–2419
Denny MW, Dowd W, Bilir L, Mach KJ (2011) Spreading the risk: small-scale body temperature variation among intertidal organisms and its implications for species persistence. J Exp Mar Biol Ecol 400:175–190
Dong Y, Williams GY (2011) Variations in cardiac performance and heat shock protein expression to thermal stress in two differently zoned limpets on a tropical rocky shore. Mar Biol 158:1223–1231
Dong Y, Miller LP, Sanders JG, Somero GN (2008) Heat-shock protein 70 (Hsp70) expression in four limpets of the Genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull 215:173–181
Dong Y, Ji TT, Meng XL, Dong SL, Sun WM (2010) Difference in thermotolerance between green and red color variants of the Japanese sea cucumber, Apostichopus japonicus Selenka: Hsp70 and heat-hardening effect. Biol Bull 218:87–94
DuBeau SF, Pan F, Tremblay GC, Bradley TM (1998) Thermal shock of salmon in vivo induces the heat shock protein hsp 70 and confers protection against osmotic shock. Aquaculture 168:311–323
Easton DP, Rutledge PS, Spotila JR (1987) Heat shock protein induction and induced thermal tolerance are independent in adult salamanders. J Exp Zool 241:263–267
Fangue NA, Mandic M, Richards JG, Schulte PM (2008) Swimming performance and energetics as a function of temperature in killifish Fundulus heteroclitus. Physiol Biochem Zool 81:389–401
Fangue NA, Osborne EJ, Todgham AE, Schulte PM (2011) The onset temperature of the heat-shock response and whole-organism thermal tolerance are tightly correlated in both laboratory-acclimated and field-acclimatized tidepool sculpins (Oligocottus maculosus). Physiol Biochem Zool 84:341–352
Feldmeth CR, Stone EA, Brown JH (1974) An increased scope for thermal tolerance upon acclimating pupfish (Cyprinodon) to cycling temperatures. J Comp Physiol 89:39–44
Gabbott PA (1983) Developmental and seasonal metabolic activities in marine molluscs. In: Hochachka PW (ed) The mollusca. Academic Press, New York, pp 165–217
Garrity SD (1984) Some adaptations of gastropods to physical stress on a tropical rocky shore. Ecology 65:559–574
Giraudoux P (2015) pgirmess: data analyses in ecology. R package version: 1.6.2
Gracey AY, Chaney ML, Boomhower JP, Tyburczy WR, Connor K, Somero GN (2008) Rhythms of gene expression in a fluctuating environment. Curr Biol 18:1501–1507
Hahn GM, Li GC (1990) Thermotolerance, thermoresistance, and thermosensitization. In: Morimoto RI, Tissieres A, Georgopoulos C (eds) Stress proteins in biology and medicine. Cold Spring Harbor Laboratory Press, New York, pp 79–100
Han GD, Zhang S, Marshall DJ, Ke CH, Dong YW (2013) Metabolic energy sensors (AMPK and SIRT1), protein carbonylation and cardiac function as biomarkers of thermal stress in an intertidal limpet: linking energetic allocation with environmental temperature during aerial emersion. J Exp Biol 216:3273–3282
Hassid WZ, Abraham S (1957) Chemical procedures for analysis of polysaccharides. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, pp 34–37
Helmuth B, Harley CDG, Halpin PM, O’Donnell M, Hofmann GE, Blanchette CA (2002) Climate change and latitudinal patterns of intertidal thermal stress. Science 298:1015–1017
Helmuth B, Russell BD, Connell SD, Dong Y, Harley CDG, Lima FP, Sará G, Williams GA, Mieszkowska N (2014) Beyond long-term averages: making biological sense of a rapidly changing world. Clim Change Responses 1:6
Hoffmann AA, Sørensen JG, Loeschcke V (2003) Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol 28:175–216
Hofmann GE, Todgham AE (2010) Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu Rev Physiol 72:127–145
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Huang X, Wang T, Ye Z, Han G, Dong Y (2015) Temperature relations of aerial and aquatic physiological performance in a mid-intertidal limpet Cellana toreuma: adaptation to rapid changes in thermal stress during emersion. Integr Zool 10:159–170
Jones SJ, Mieszkowska N, Wethey DS (2009) Linking thermal tolerances and biogeography: Mytilus edulis (L.) at its southern limit on the east coast of the United States. Biol Bull 217:73–85
Kingsolver JG, Ragland GJ, Diamond SE (2009) Evolution in a constant environment: thermal fluctuations and thermal sensitivity of laboratory and field populations of Manduca sexta. Evolution 63:537–541
Krebs RA, Loeschcke V (1994) Costs and benefits of activation of the heat-shock response in Drosophila melanogaster. Funct Ecol 8:730–737
Krebs RA, Roberts SP, Bettencourt BR, Feder ME (2001) Changes in thermotolerance and Hsp70 expression with domestication in Drosophila melanogaster. J Evol Biol 14:75–82
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186
Lambert Y, Dutil JD (2000) Energetic consequences of reproduction in Atlantic cod (Gadus morhua) in relation to spawning level of somatic energy reserves. Can J Fish Aquat Sci 57:815–825
Lawrence JM (1976) Patterns of lipid storage in post-metamorphic marine invertebrates. Am Zool 16:747–762
Li Y, Qin JG, Abbott CA, Li X, Benkendorff K (2007) Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: an explanation for summer mortality in Pacific oysters. Am J Physiol Regul Integr 293:2353–2362
Lindberg DR, Pearse JS (1990) Experimental manipulation of shell color and morphology of the limpets Lottia asmi (Middendorff) and Lottia digitalis (Rathke) (Mollusca: Patellogastropoda). J Exp Mar Biol Ecol 140:173–185
Loeschcke V, Hoffmann AA (2007) Consequences of heat hardening on a field fitness component in Drosophila depend on environmental temperature. Am Nat 169:175–183
Loeschcke V, Krebs RA, Barker J (1994) Genetic variation for resistance and acclimation to high temperature stress in Drosophila buzzatii. Biol J Linn Soc Lond 52:83–92
Lurman G, Blaser T, Lamare M, Tan K, Pörtner H, Peck LS, Morley SA (2010) Ultrastructure of pedal muscle as a function of temperature in nacellid limpets. Mar Biol 157:1705–1712
Maness JD, Hutchison VH (1980) Acute adjustment of thermal tolerance in vertebrate ectotherms following exposure to critical thermal maxima. J Therm Biol 5:225–233
Marshall DJ, McQuaid CD (1991) Metabolic rate depression in a marine pulmonate snail: pre-adaptation for a terrestrial existence? Oecologia 88:274–276
Marshall DJ, McQuaid CD (1993) Effects of hypoxia and hyposalinity on the heart beat of the intertidal limpets Patella granvlaris (Prosobranchia) and Siphonaria capensis (Pulmonata). Comp Biochem Phys A 106:65–68
Marshall KE, Sinclair BJ (2010) Repeated stress exposure results in a survival-reproduction trade-off in Drosophila melanogaster. Proc Biol Sci 277:963–969
Marshall DJ, Dong Y, McQuaid CD, Williams GA (2011) Thermal adaptation in the intertidal snail Echinolittorina malaccana contradicts current theory by revealing the crucial roles of resting metabolism. J Exp Biol 214:3649–3657
McMahon RF (1990) Thermal tolerance, evaporative water loss, air-water oxygen consumption and zonation of intertidal prosobranchs: a new synthesis. Hydrobiologia 193:241–260
Middlebrook R, Hoegh-Guldberg O, Leggat W (2008) The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J Exp Biol 211:1050–1056
Miller LP, Harley CDG, Denny MW (2009) The role of temperature and desiccation stress in limiting the local-scale distribution of the owl limpet, Lottia gigantea. Funct Ecol 23:756–767
Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30:429–440
Petes LE, Menge BA, Harris AL (2008) Intertidal mussels exhibit energetic trade-offs between reproduction and stress resistance. Ecol Monogr 78:387–402
Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team (2013) nlme: linear and nonlinear mixed effects models. R package version: 3.1-103
Podrabsky JE, Somero GN (2004) Changes in gene expression associated with acclimation to constant temperatures and fluctuating daily temperatures in an annual killifish Austrofundulus limnaeus. J Exp Biol 207:2237–2254
Pöhlmann K, Koenigstein S, Alter K, Abele D, Held C (2011) Heat-shock response and antioxidant defense during air exposure in Patagonian shallow-water limpets from different climatic habitats. Cell Stress Chaperon 16:621–632
Pörtner H-O (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A 132:739–761
Pörtner H-O (2010) Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893
R Development Core Team (2012) R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2012; http://www.R-project.org)
Santini G, Chelazzi G (1995) Glycogen content and rates of depletion in two limpets with different foraging regimes. Comp Biochem Physiol 111A:271–277
Santini G, Bianchi T, Chelazzi G (2002) Metabolic responses to food deprivation in two limpets with different foraging regimes, revealed by recording of cardiac activity. J Zool Long 256:11–15
Segal E (1956) Microgeographic variation as thermal acclimation in an intertidal mollusc. Biol Bull 111:129–152
Simpson RD (1982) Reproduction and lipids in the sub-Antarctic limpet Nacella (Patinigera) macquariensis Finlay, 1927. J Exp Mar Biol Ecol 56:33–48
Sinclair BJ, Nelson S, Nilson TL, Roberts SP, Gibbs AG (2007) The effect of selection for desiccation resistance on cold tolerance of Drosophila melanogaster. Physiol Entomol 32:322–327
Sinclair BJ, Ferguson LV, Salehipour-Shirazi G, Macmillan HA (2013) Cross-tolerance and cross-talk in the cold: relating low temperatures to desiccation and immune stress in insects. Integr Comp Biol 53:545–556
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Smith RL, Paul AJ, Paul JM (1990) Seasonal changes in energy and the energy cost of spawning in Gulf of Alaska Pacific cod. J Fish Biol 36:307–316
Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA (2012) Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res 79:1–15
Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comp Biol 42:780–789
Somero GN (2012) The physiology of global change: linking patterns to mechanisms. Annu Rev Mar Sci 4:39–61
Sørensen JG, Kristensen TN, Loeschcke V (2003) The evolutionary and ecological role of heat shock proteins. Ecol Lett 6:1025–1037
Sørensen CH, Toft S, Kritstensen TN (2012) Cold-acclimation increases the predatory efficiency of the aphidophagous coccinellid Adalia bipunctata. Biol Control 65:87–94
Stenseng E, Braby CE, Somero GN (2005) Evolutionary and acclimation induced variation in the thermal limits of heart function in congeneric marine snails (genus Tegula): implications for vertical zonation. Biol Bull 208:138–144
Stillman JH (2003) Acclimation capacity underlies susceptibility to climate change. Science 301:65
Stillman J, Somero G (1996) Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): correlation of physiology, biochemistry and morphology with vertical distribution. J Exp Biol 199:1845–1855
Todgham AE, Schulte PM, Iwama GK (2005) Cross-tolerance in the tidepool sculpin: the role of heat shock proteins. Physiol Biochem Zool 78:133–144
Todgham AE, Iwama GK, Schulte PM (2006) Effects of the natural tidal cycle and artificial temperature cycling on Hsp levels in the tidepool sculpin Oligocottus maculosus. Physiol Biochem Zool 79:1033–1045
Tomanek L (2008) The importance of physiological limits in determining biogeographical range shifts due to global climate change: the heat-shock response. Physiol Biochem Zool 81:709–717
Tomanek L, Helmuth B (2002) Physiological ecology of rocky intertidal organisms: a synergy of concepts. Integr Comp Biol 42:771–775
Tomanek L, Sanford E (2003) Heat-shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (genus: Tegula). Biol Bull 205:276–284
Voltzow J (1994) Gastropoda: Prosobranchia. In: Harrison FW, Kohn AJ (eds) Microscopic anatomy of invertebrates, volume 5: mollusca. Wiley-Liss, New York, pp 111–252
Wethey DS, Woodin SA (2008) Ecological hindcasting of biogeographic responses to climate change in the European intertidal zone. Hydrobiologia 606:139–151
Widdows J (1976) Physiological adaptation of Mytilus edulis to cyclic temperatures. J Comp Physiol 105:115–128
Williams GA, Morritt D (1995) Habitat partitioning and thermal tolerance in a tropical limpet, Cellana grata. Mar Ecol Prog Ser 124:89–103
Williams GA, De Pirro M, Leung KMY, Morritt D (2005) Physiological responses to heat stress on a tropical shore, the benefits of mushrooming behaviour in the limpet Cellana grata. Mar Ecol Prog Ser 292:213–224
Wolcott TG (1973) Physiological ecology and intertidal zonation in limpets (Acmaea): a critical look at “limiting factors”. Biol Bull 145:389–422
Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99:673–686
Zuur A, Leno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We would like to thank Dr. Jonathon Stillman for his helpful input in heart rate analysis and use of his heart rate setup as well as Dr. Lars Tomanek for his helpful discussions on the manuscript. We would also like to thank Dr. Nate Miller for his assistance with the GAMM analyses and Erin Flynn for help with the statistical analyses. This work was supported by San Francisco State University to AET, the University of California Agricultural Experiment Station (Grant Number CA-D-ASC-2252-H to A.E.T.), and a CSU Council on Oceans Affairs, Science and Technology (COAST) student scholarship to CP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: H. Pörtner.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Examples of limpet heart rate traces during the Day 2, lethal temperature ramp from 13 to 48 °C at a rate of 6 °C h−1. Each panel portrays what a limpet trace might look like with 0 (panel 1), 1 (panel 2), 2 (panel 3), and 3 breaks (panel 4) in heart rate. Breaks in heart rate were defined as when heart rate decreased followed by an additional increase in activity. Summer laboratory-acclimated limpets had 1, 2, or 3 breaks in heart rate, while winter laboratory-acclimated limpets experienced 0, 1, and 2 breaks in heart rate (PDF 2598 kb)
Rights and permissions
About this article
Cite this article
Pasparakis, C., Davis, B.E. & Todgham, A.E. Role of sequential low-tide-period conditions on the thermal physiology of summer and winter laboratory-acclimated fingered limpets, Lottia digitalis . Mar Biol 163, 23 (2016). https://doi.org/10.1007/s00227-015-2779-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-015-2779-5