Abstract
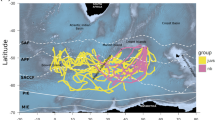
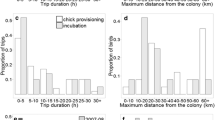
Seabird populations contain large numbers of immatures––in some instances comprising >50% of the fully grown adults in the population. These birds are significant components of marine food webs and may contribute to compensatory recruitment and dispersal, but remain severely understudied. Here, we use GPS-PTTs, radio-tracking and analysis of stable carbon (δ13C) and nitrogen (δ15N) isotopes to investigate the movements and foraging ecology of immature seabirds. Our study focussed on immature northern gannets Morus bassanus aged 2–4 attending non-breeding aggregations alongside a large breeding colony. GPS-PTT tracking of five birds revealed that immatures have the ability to disperse widely during the breeding season, with some individuals potentially prospecting at other colonies. Overall, however, immatures were faithful to the colony of capture. During returns to the focal colony, immatures acted as central place foragers, conducted looping and commuting flights, and analysis of the variance in first-passage time revealed evidence of area-restricted search (ARS) behaviour. In addition, stable carbon (δ13C) and nitrogen (δ15N) isotope analyses indicate that immatures were isotopically segregated from breeders. Our findings provide insights into the foraging, prospecting and dispersal behaviour of immature seabirds, which may have important implications for understanding seabird ecology and conservation.



Similar content being viewed by others
References
Bearhop S, Adams CE, Waldron S, Fuller RA, Macleod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012
Boulinier T, McCoy KD, Yoccoz NG, Gasparini J, Tveraa T (2008) Public information affects breeding dispersal in a colonial bird: kittiwakes cue on neighbours. Biol Lett 4:538–540
Brooke MD (2004) Food consumption of the world’s seabirds. Proc R Soc B 271:S246–S248
Catry P, Furness RW (1999) The influence of adult age on territorial attendance by breeding great skuas Catharacta skua: an experimental study. J Avian Biol 30:399–406
Courchamp F, Ludek B, Gascoigne J (2008) Allee effects in ecology and conservation. Oxford University Press, Oxford
Coyne MS, Godley BJ (2005) Satellite Tracking and Analysis Tool (STAT): an integrated system for archiving, analyzing and mapping animal tracking data. Mar Ecol Prog Ser 301:1–7
Dittmann T, Zinsmeister D, Becker PH (2005) Dispersal decisions: common terns, Sterna hirundo, choose between colonies during prospecting. Anim Behav 70:13–20
Fauchald P, Tveraa T (2003) Using first-passage time in the analysis of area-restricted search and habitat selection. Ecology 84:282–288
Garthe S, Montevecchi WA, Chapdelaine G, Rail JF, Hedd A (2007) Contrasting foraging tactics by northern gannets (Sula bassana) breeding in different oceanographic domains with different prey fields. Mar Biol 151:687–694
Guilford T, Meade J, Willis J, Phillips RA, Boyle D, Roberts S, Collett M, Freeman R, Perrins CM (2009) Migration and stopover in a small pelagic seabird, the Manx shearwater Puffinus puffinus: insights from machine learning. Proc R Soc B 276:1215–1223
Halley DJ, Harris MP (1993) Intercolony movement and behaviour of immature guillemots Uria aalge. Ibis 135:264–270
Halley DJ, Harris MP, Wanless S (1995) Colony attendance patterns and recruitment in immature Common Murres (Uria aalge). Auk 112:947–957
Hamer KC, Phillips RA, Wanless S, Harris MP, Wood AG (2000) Foraging ranges, diets and feeding locations of gannets Morus bassanus in the North Sea: evidence from satellite telemetry. Mar Ecol Prog Ser 200:257–264
Hamer KC, Phillips RA, Hill JK, Wanless S, Wood AG (2001) Contrasting foraging strategies of gannets Morus bassanus at two North Atlantic colonies: foraging trip duration and foraging area fidelity. Mar Ecol Prog Ser 224:283–290
Hamer KC, Humphreys EM, Magalhaes MC, Garthe S, Hennicke G, Peters G, Gremillet D, Wanless S (2009) Fine-scale foraging behaviour of a medium-ranging marine predator. J Anim Ecol 78:880–889
Hatchwell BJ, Birkhead TR (1991) Population-dynamics of common guillemots Uria-Aalge on Skomer Island, Wales. Ornis Scand 22:55–59
Hobson KA (2005) Using stable isotopes to trace long-distance dispersal in birds and other taxa. Divers Distrib 11:157–164
Huyvaert KP, Anderson DJ (2004) Limited dispersal by Nazca boobies Sula granti. J Avian Biol 35:46–53
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461
Ismar SMH, Hunter C, Lay K, Ward-Smith T, Wilson PR, Hauber ME (2010) A virgin flight across the Tasman Sea? Satellite tracking of post-fledging movement in the Australasian Gannet Morus serrator. J Ornithol 151:755–759
Klomp NI, Furness RW (1992) Non-breeders as a Buffer against Environmental-Stress––declines in numbers of great Skuas on Foula, Shetland, and prediction of future recruitment. J Appl Ecol 29:341–348
Kokko H, Lopez-Sepulcre A (2006) From individual dispersal to species ranges: perspectives for a changing world. Science 313:789–791
Lewis S, Benvenuti S, Dall’Antonia L, Griffiths R, Money L, Sherratt TN, Wanless S, Hamer KC (2002) Sex-specific foraging behaviour in a monomorphic seabird. Proc R Soc B 269:1687–1693
Louzao M, Hyrenbach KD, Arcos JM, Abello P, De Sola LG, Oro D (2006) Oceanographic habitat of an endangered mediterranean procellariiform: implications for marine protected areas. Ecol Appl 16:1683–1695
McCoy KD, Boulinier T, Tirard C (2005) Comparative host-parasite population structures: disentangling prospecting and dispersal in the black-legged kittiwake Rissa tridactyla. Mol Ecol 14:2825–2838
Mitchell PI, Newton SF, Ratcliffe N, Dun TE (2004) Seabird populations of Britain and Ireland. Christopher Helm, London
Nelson B (2002) The atlantic gannet. Fenix Books Ltd, Norfolk
Pinaud D (2008) Quantifying search effort of moving animals at several spatial scales using first-passage time analysis: effect of the structure of environment and tracking systems. J Appl Ecol 45:91–99
Ropert-Coudert Y, Wilson RP (2005) Trends and perspectives in animal-attached remote sensing. Front Ecol Environ 3:437–444
Serrano D, Tella JL (2003) Dispersal within a spatially structured population of lesser kestrels: the role of spatial isolation and conspecific attraction. J Anim Ecol 72:400–410
Votier SC, Bearhop S, Fyfe R, Furness RW (2008a) Temporal and spatial variation in the diet of a marine top predator––links with commercial fisheries. Mar Ecol Prog Ser 367:223–232
Votier SC, Birkhead TR, Oro D, Trinder M, Grantham MJ, Clark JA, McCleery RH, Hatchwell BJ (2008b) Recruitment and survival of immature seabirds in relation to oil spills and climate variability. J Anim Ecol 77:974–983
Votier SC, Bearhop S, Witt MJ, Inger R, Thompson D, Newton J (2010) Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems. J Appl Ecol 47:487–497
Weimerskirch H (1992) Reproductive effort in long-lived birds––age-specific patterns of condition, reproduction and survival in the wandering albatross. Oikos 64:464–473
Weimerskirch H (2002) Seabird demography and its relationship with the marine environment. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, London, pp 115–135
Weimerskirch H (2007) Are seabirds foraging for unpredictable resources? Deep Sea Res Part 2 Top Stud Oceanogr 54:211–223
Weimerskirch H, Akesson S, Pinaud D (2006) Postnatal dispersal of wandering albatrosses Diomedea exulans: implications for the conservation of the species. J Avian Biol 37:23–28
Weimerskirch H, Pinaud D, Pawlowski F, Bost CA (2007) Does prey capture induce area-restricted search? A fine-scale study using GPS in a marine predator, the wandering albatross. Am Nat 170:734–743
Acknowledgments
We would like to thank Greg & Lisa Morgan, Tim Brooke at VentureJet, Anthony Bicknell, Valentina Lauria, Carrie Gunn, James Waggitt, Claudia Stauss and Simon Rundle for help in field, laboratory and for comment on the manuscript. The Royal Society for the Protection of Birds granted permission to work on Grassholm. Device attachment was conducted with permission of the Countryside Council for Wales. SCV was funded by a NERC New Investigators Grant (NE/G001014/1), WJG funded by a studentship from the Peninsula Research Institute for Marine Renewable Energy and SP funded by EU INTERREG Project CHARM III.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. E. Hauber.
Rights and permissions
About this article
Cite this article
Votier, S.C., Grecian, W.J., Patrick, S. et al. Inter-colony movements, at-sea behaviour and foraging in an immature seabird: results from GPS-PPT tracking, radio-tracking and stable isotope analysis. Mar Biol 158, 355–362 (2011). https://doi.org/10.1007/s00227-010-1563-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1563-9