Abstract
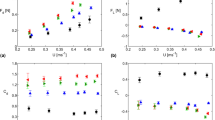
Nearly all organisms show directional bias in sensitivity to environmental signals. In this study, the behavioral sensitivity of a common estuarine copepod, Acartia tonsa, varies significantly with respect to their orientation to a well-characterized fluid mechanical signal. Maximum sensitivity occurs at an angle of 25°–30° and lowest sensitivity occurs at angles of 60°–90° relative to the source. These results support the hypothesis that copepods are not uniformly sensitive to fluid signals and show directional bias in mechanosensitivity. The data also show that large copepods initiate their escape reaction further from the source than small copepods. There is, however, an uncharacteristically large increase in sensitivity at the transition between the nauplii and C1 stage despite being similar in size. This suggests that the mechanosensory system of the naupliar stages is less sensitive to fluid signals and helps to explain the higher predation rates experienced by nauplii.






Similar content being viewed by others
References
Andrews JC (1983) Deformation of the active space in the low Reynolds number feeding current of calanoid copepods. Can J Fish Aquat Sci 40:1293–1302
Anjum F, Turni H, Mulder PGH, van de Burg J, Brecht M (2006) Tactile guidance of prey capture in Etruscan shrews. PNAS 103:16544–16549
Ball EE, Cowan N (1977) Ultrastructure of the antennal sensilla of Acetes (Crustace, Decapod, Natantia, Sergestidae). Philos Trans R Soc Lond B Biol Sci 277:429–456
Batchelor GK (1968) An introduction to fluid dynamics. Cambridge University Press, Cambridge, p 615
Batty RS, Blaxter JHS, Richard JM (1990) Light intensity and the feeding behaviour of herring, Clupea harengus. Mar Biol 107:383–388
Boxshall GA, Yen J, Strickler JR (1997) Functional significance of the sexual dimorphism in the array of setation elements along the antennules of Euchaeta rimana Bradford. Bull Mar Sci 61:387–398
Brooks JL (1946) Cyclomorphosis in Daphnia I. An analysis of D. retrocurva and D. galeata. Ecol Monographs 16:409–447
Browman HI, Obrien WJ (1992a) The ontogeny of search behavior in the white crappie Pomoxis-annularis. Exp Biol Fish 34(2):181–195
Browman HI, Obrien WJ (1992b) Foraging and prey search behavior of golden Shinner (Notemigonus-crysoleucas) larvae. Can J Fish Aquat Sci 49(4):813–819
Bundy MH, Paffenhofer G-A (1996) Analysis of flow fields associated with freely swimming calanoid copepods. Mar Ecol Prog Ser 133:99–113
Dagan D, Parnas I (1970) Giant Fibre and small fibre pathways involved in the evasive response of the American cockroach, Periplaneta americana. J Exp Biol 52:313–324
Doall MH, Strickler JR, Fields DM, Yen J (2002) Mapping the attack volume of a free-swimming planktonic copepod, Euchaeta rimana. Mar Biol 140:871–879
Enright JT, Hamner WM (1967) Vertical diurnal migration and endogenous rhythmicity. Science 25:937–941
Fields DM (2000) Characteristics of the high frequency escape reactions of Oithona sp. Mar Freshw Behav Physiol 34:21–35
Fields DM, Yen J (1993) Outer limits and inner structure: the 3-dimensional flow field of Pleuromamma xiphias (Copepoda). Bull Mar Sci 53:84–95
Fields DM, Yen J (1996) The escape behavior of Pleuromamma xiphias from a quantifiable fluid mechanical disturbance. In: Lenz PH, Hartline DK, Purcell JE, Macmillan DL (eds) Zooplankton: Sensory ecology and physiology. Gordan and Breach Publ, Amsterdam, pp 323–340
Fields DM, Yen J (1997a) The escape behavior of marine copepods in response to a quantifiable fluid mechanical disturbance. J Plankton Res 19:1289–1304
Fields DM, Yen J (1997b) Implication of copepod feeding currents on the spatial orientation of their prey. J Plankton Res 19:79–85
Fields DM, Yen J (2002) Fluid mechanosensory stimulation of behavior from a planktonic marine copepod Euchaeta rimana Bradford. J Plankton Res 24:747–755
Fields DM, Shaeffer DS, Weissburg MJ (2002) Mechanical and neural responses from the mechanosensory hairs on the antennule of Gaussia princeps. Mar Ecol Prog Ser 227:173–186
Fields DM, Weissburg MJ (2004) Rapid depolarization rates from the antennules of copepods. J Comp Phys A 190:877–882
Fields DM, Weissburg MJ (2005) Evolutionary and ecological significance of mechanosensory morphology: copepods as a model system. Mar Ecol Prog Ser 287:269–274
Herberholz J, Sen MN, Edwards DH (2004) Escape behavior and escape circuit activation in juvenile crayfish during prey–predator interactions. J Exp Biol 207:1855–1863
Humphrey JAC, Barth FG (2007) Medium flow-sensing hairs: biomechanics and models. In: Casas J, Simpson SJ (eds) Insect mechanics and control: Adv insect physiol, vol 34, pp 1–80
Huys R, Boxshall GA (1991) Copepod evolution. Ray Society, London
Insausti TC, Lazzari CR, Casas J (2008) The terminal abdominal ganglion of the wood cricket Nemobius sylvestris. J Morphol 269:1539–1551
Janssen J (1981) Searching for zooplankton just outside of Snell’s window. Limnol Oceanogr 26:1168–1171
Jiang H, Strickler JR (2005) Mass density contrast in relation to the feeding currents in calanoid copepods. J Plankton Res 27:1003–1012
Jonsson PR, Andre C, Lindegarth M (1991) Swimming behavior of marine bivalve larvae in a flume boundry-layer flow: evidence for near-bottom confinement. Mar Ecol Prog Ser 79:67–76
Kessler JO (1985) Hydrodynamic focusing of motile algal cells. Nature 313:220–318
Kiørboe T, Saiz E, Visser AW (1999) Hydrodynamic signal perception in the copepod Acartia tonsa. Mar Ecol Prog Ser 179:97–111
Kumagai T, Shimozawa T, Baba Y (1998) The shape of wind receptor hairs of cricket and cockroach. J Comp Physiol A 183:187–192
Kurbjeweit F, Buchholz C (1991) Structure and suspected functions of antennular sensilla and pores of three Arctic copepods, Calanus glacialis, Metridia longa, Paraeuchaeta norvegica. Meeresforsch 33:168–182
Laverack MS (1976) External proprioceptors. In: Mill PJ (ed) Structure and function of proprioceptors in invertebrates. Chapman & Hall, London, pp 1–63
Lenz PH, Hartline DK (1999) Reaction times and force production during escape behavior of a calanoid copepod, Undinula vulgaris. Mar Biol 133:249–258
Lenz PH, Hower AE, Hartline DK (2004) Force production during pereiopod power strokes in Calanus finmarchicus. J Mar Syst 49:133–144
Pennington JT, Strathmann RR (1990) Consequences of the calcite skeletons of planktonic echinoderm larvae for orientation, swimming, and shape. Biol Bull 179:121–133
Roberts AM (1970) Geotaxis in motile micro-organisms. J Exp Biol 53:687–699
Shimozawa T, Kumagai T, Babba Y (1998) Structural scaling and functional design of cercal wind-receptor hairs of cricket. J Comp Physiol A 183:171–186
Strickler JR (1975) Swimming of planktonic Cyclops species (Copepoda, Crustacea): pattern, movements and their control. In: Wu TY-T, Brokaw CJ, Brennan C (eds) Swimming and flying in nature. Plenum Press, New York, pp 599–613
Tazaki K (1977) Nervous responses from mechanosensory hairs on the antennal flagellum in the lobster Homarus gammarus (L.). Mar Behav Physiol 5:1–18
Thetmeyer H, Kils U (1995) To see and not be seen: the visibility of predator and prey with respect to feeding behaviour. Mar Ecol Prog Ser 126:1–8
Vogel S (1981) Life in moving fluids. Princeton University Press, Princeton
Waggett RJ, Buskey EJ (2007) Copepod escape behavior in non-turbulent and turbulent hydrodynamic regimes. Mar Ecol Prog Ser 334:193–198
Weatherby TM, Davis AD, Hartline DK, Lenz PH (2000) The need for speed. II. Myelin in calanoid copepods. J Comp Physiol 186:347–357
Weise K (1976) Mechanoreception for near-field water displacements in crayfish. J Neurophysiol (Bethesda) 39:816–833
Yen J, Lenz PH, Gassie DV, Hartline DK (1992) Mechanoreception in marine copepods: electrophysiological studies of the first antennae. J Plankton Res 14:495–512
Acknowledgments
Thanks to Donald Webster for his constructive criticism. Support for this work was provided by NSF/IOS #0718832 awarded to DM Fields.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. A. Peck.
Rights and permissions
About this article
Cite this article
Fields, D.M. Orientation affects the sensitivity of Acartia tonsa to fluid mechanical signals. Mar Biol 157, 505–514 (2010). https://doi.org/10.1007/s00227-009-1336-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1336-5