Abstract
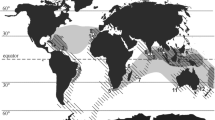
Stable isotopes (δ18O and δ13C) were measured in successive chambers of the aragonitic shells of the small deep-sea squid Spirula spirula (Linnaeus 1758) (class Cephalopoda, subclass Coleoidea, order Sepioidea, family Spirulidae) to determine whether their depth distributions change with age. The spiral shells, ranging in diameter from 18 to 23 mm (30–38 chambers), were collected between 2000 and 2006 from beaches in six widely separated locations in three oceans, the Atlantic (Tobago and Canary Islands), Indian (Madagascar, Maldives, and Perth, Australia), and Pacific Oceans (Ulladulla, Australia). The patterns for both isotopes were highly correlated in specimens from all six sites. The δ18O data suggest that after hatching at depths >1,000 m at temperatures of 4–6°C, the squid migrate into shallower, warmer waters at 12–14°C at depths of 400–600 m. Subsequently, the increasing δ18O values suggest a migration back into somewhat cooler, deeper habitats. The δ13C values also revealed three ontogenetic stages in all six specimens, including a major shift from positive to negative values, which probably corresponds to sexual maturation, the initiation of reproduction, and concomitant changes in diet. In three of the six specimens (from Tobago, Canary Islands, and Maldives) a fourth embryonic stage (not detected in the oxygen data) was accompanied by markedly less positive δ13C values in the first few chambers. These data, combined with the scanty life history information from previous studies of S. spirula, can be used to compare the habitat requirements of related extant and fossil cephalopod genera.





Similar content being viewed by others
References
Appellöff A (1893) Die Schalen von Sepia, Spirula und Nautilus. Studium über den Bau und das Wachstum. K svenska Vetensk Akad. Hand Stockholm 25:1–106
Auclair A-C, Lecuyer C, Bucher H, Sheppard SMF (2004) Carbon and oxygen isotope composition of Nautilus macromphalus: a record of thermocline waters off New Caledonia. Chem Geol 207:91–100
Bandel K (1982) Morphologie und Bildung der frühontogenetischen Gehäuse bei conchiferen Mollusken. Facies 7:1–198
Böggild OB (1930) The shell structure of the molluscs. Kgl danske Vidensk Selsk Skr nat og mat 9:233–326
Bruun AF (1943) The biology of Spirula spirula (L.). Dana Report 24:1–48
Cherel Y, Hobson KA (2005) Stable isotopes, beaks and predators: a new tool to study the trophic ecology of cephalopods, including giant and colossal squids. Proc R Soc B 272:1601–1607
Chun C (1910) Spirula australis Lam. Ber Math Phys Kl K Sächs Ges Wiss Leipzig 62:171–188
Chun C (1915) Die Cephalopoda. 2 Teil: Myopsida, Octopoda. Wiss Ergeb Dt Tiefsee Exp 18:414–476
Clarke MR (1966) A review of the systematics and ecology of oceanic squids. Adv Mar Biol 4:91–300
Clarke MR (1969) Cephalopoda collected on the SOND cruise. J Mar Biol Ass UK 49:961–976
Clarke MR (1970) Growth and development of Spirula spirula. J Mar Biol Ass UK 50:53–64
Clarke MR (1986) A handbook for the identification of cephalopod beaks. Clarendon Press, Oxford
Cochran JK, Rye DM, Landman NH (1981) Growth rate and habitat of Nautilus pompilius inferred from radioactive and stable isotope studies. Paleobiology 7:469–480
Dauphin Y (1976) Microstructure des coquilles de céphalopodes. I. Spirula spirula L. (Dibranchiata, Decapoda). Bull Mus Natn Hist Nat 382:197–238
Dauphin Y (2001a) Caractéristiques de la phase organique soluble des tests aragonitiques des trios genres de cephalopods actuels. N Jb Geol Paläont Mh 2001(2):103–123
Dauphin Y (2001b) Nanostructures de la nacre des tests de cephalopods actuels. Paläont Z 75:113–122
Doguzhaeva LA (1996) Two Early Cretaceous spirulid coleoids of the North-Western Caucasus: their shell ultrastructure and evolutionary implications. Palaeontology 39:681–709
Doguzhaeva LA (2000) The evolutionary morphology of siphonal tube, in Spirulida (Cephalopoda, Coleoidea). Rev Paléobiol Vol Spéc 8:83–94
Donovan DT (1977) Evolution of the dibranchiate cephalopoda. In: Nixon M, Messenger JB (eds) The biology of cephalopods. Symp Zool Soc London, vol 38, Academic Press, London, pp 15–48
Eichler R, Ristedt H (1966) Untersuchungen zur Frühontogenie von Nautilus pompilius (Linné). Paläont Z 40:173–191
Goodwin DH, Schöne BR, Dettman DL (2003) Resolution and fidelity of oxygen isotopes as palaeotemperature proxies in bivalve mollusc shell: models and observations. Palaios 18:110–125
Goud J (1985) Spirula spirula, een inktvis uit de diepzee. Vita marina, Zeebiol doku, pp 35–42
Grossman EL, Ku T (1986) Oxygen and carbon isotope fractionation in biogenic aragonite: temperature effects. Chem Geol 59:59–74
Hobson KA, Cherel Y (2006) Isotopic reconstruction of marine food webs using cephalopod beaks: new insight from captively raised Sepia officinalis. Can J Zool 84:766–770
Joubin L (1995) Cephalopods from the scientific expeditions of Prince Albert I of Monaco. Vol. I, parts 1-III; Vol. 2 parts III-IV. Smithsonian Institution Libraries and Nati Sci Found, Washington, DC
Kerr JG (1931) Notes upon the Dana specimens of Spirula and upon certain problems of cephalopod morphology. Dana Report 8:1–34
Keupp H (2000) Ammoniten: Paläobiologische Erfolgsspiralen. Thorbecke Verlag, Stuttgart
Landman NH, Rye DM, Shelton KL (1983) Early ontogeny of Eutrephoceras compared to Recent Nautilus and Mesozoic ammonites: evidence from shell morphology and light stable isotopes. Paleobiology 9:269–279
Landman NH, Cochran JK, Rye DM, Tanabe K, Arnold JM (1994) Early life history of Nautilus: evidence from isotopic analysis of aquarium reared specimens. Paleobiology 20:40–51
LEVITUS 94 (1994) World Ocean Atlas. http://ingrid.ldeo.columbia.edu/SOURCES/.LEVITUS94/
Lindgren AR, Giribet G, Nishiguchi MK (2004) A combined approach to the phylogeny of Cephalopoda (Mollusca). Cladistics 20:454–486
Linnaeus C (1758) Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Edito decimal, reformata. Holmiae: Laurentii Salvii vol 1, 824, p 710
Lu CC (1998) Order Sepioidea. Chap. 13. In: Beesley PL, Ross GJB, Wells A (eds) Mollusca. The southern synthesis. Fauna of Australia. Part A, vol. 5. CSIRO Publishing, Melbourne, pp 504–514
McConnaughey TA, Burdett J, Whelan JF, Paull CK (1997) Carbon isotopes in biological carbonates: respiration and photosynthesis. Geochim Cosmochim Acta 61:611–622
Mutvei H (1964) On the shells of Nautilus and Spirula with notes on the shell secretion in non-cephalopod molluscs. Ark Zool 16:221–278
Mutvei H, Donovan DT (2006) Siphuncular structure in some fossil coleoids and recent Spirula. Palaeontology 49:685–691
Naef A (1922) Die Fossilen Tintenfische. Gustav Fischer, Jena
Nixon M, Dilly PN (1977) Sucker surfaces and prey capture. In: Nixon M, Messenger JB (eds) The biology of cephalopods. Symp Zool Soc London, vol 38. Academic Press, London, pp 447–511
Norman M (2007) Australian biological resources study. Species Bank. http://www.environment.gov.au/cgi-bin/species-bank/sbank-treatment.pl?id=77088
Pelseneer P (1895) Observations on Spirula. Bull Sci Fr Belg 26:1–55
Rexfort A, Mutterlose J (2006) Stable isotope records from Sepia officinalis - a key to understand the ecology of belemnites? Earth Planet Sci Lett 247:212–221
Rosa R, Costa PR, Bandarra N, Nunes ML (2005) Changes in tissue. Biochemical composition and energy reserves associated with sexual maturation in the ommastrephid squids Illex coindetii and Todaropsis eblanae. Biol Bull 208:100–113
Schmidt J (1922) Live specimens of Spirula. Nature 110, No. 2271, pp 788–790
Taylor BE, Ward PD (1983) Isotopic studies of Nautilus macromphalus Sowerby (New Caledonia) and Nautilus pompilius L. (Fiji). Palaeogeogr Palaeoclimatol Palaeoecol 41:1–16
Turek R (1933) Chemisch-analytische Untersuchungen an Molluskenschalen. Arch Naturgesch Leipzig Z Syst Zool 2:301–202
Warnke K, Keupp H (2005) Spirula—a window to the embryonic development of ammonoids? Morphological and molecular indications for a palaeontological hypothesis. Facies 51:60–65
Warnke K, Plötner J, Santana JI, Rueda MJ, Llinás O (2003) Reflections on the phylogenetic position of Spirula (Cephalopoda): preliminary evidence from the 18S ribosomal RNA gene. Berliner Paläobiol Abh 03:253–260
Young JZ (1977) Brain, behaviour and evolution of cephalopods. Symp Zool Soc Lond 38:377–434
Young RE, Vecchione M, Donovan D (1998) The evolution of coleoid cephalopods and their present biodiversity and ecology. S Afr J Mar Sci 20:393–420
Acknowledgments
We are grateful to Ortwin Schultz, Peter Sziemer, Franz Topka (all Natural History Museum Vienna) and Jens Hartmann (University of Hamburg) for providing Spirula spirula specimens. We thank Vera Hammer (Natural History Vienna) for geochemical analysis of the shell material. Two anonymous reviewers helped to improve the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lukeneder, A., Harzhauser, M., Müllegger, S. et al. Stable isotopes (δ18O and δ13C) in Spirula spirula shells from three major oceans indicate developmental changes paralleling depth distributions. Mar Biol 154, 175–182 (2008). https://doi.org/10.1007/s00227-008-0911-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-0911-5