Abstract
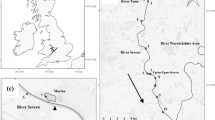
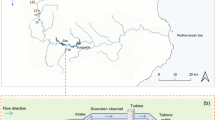
Dense populations of the fiddler crab Uca minax (Le Conte 1855) are common along tidally influenced freshwater rivers and streams >50 km from the sea. Adults do not migrate from inland sites to release larvae, but instead release them directly into an environment where the zoeae cannot survive. Laboratory salinity tolerance experiments were used to determine how long larvae from the inland-most population of U. minax along the Pee Dee River, South Carolina, USA can survive zero salinity compared to larvae from a brackish water population (salinity 5) near the mouth of Winyah Bay in the same estuary. Larvae from the brackish water population were also exposed to a salinity of 5 and their survival tracked. These experiments were conducted from May to August 2004 and 2005. To determine if inland larvae suffered significant mortality in transit due to salinity stress, current profiles were measured in the field and used to model the time taken by a larva using ebb-tide transport to travel to permissive salinities. The laboratory tolerance experiments showed that larvae from the inland freshwater population had LT50’s of 4–5 days at 0 salinity, which were significantly longer than those of the brackish water zoeae (2–3 days). Zoeae from the brackish water population survived for at least one larval molt at a salinity of 5 with LT 50’s of ∼12 days. Estimated travel times to reach permissive salinities from the inland-most population based on current profiles were 3–5 days for larvae using night-time only ebb-tide transport and 1.5–2.5 days for those using ebb-tide transport both day and night. Previously published field data indicate that U. minax larvae do use both day- and night-time ebb-tide transport, and are found in high densities in the water column during the day. These results lead to the conclusion that U. minax stage I zoeae do not undergo significant salinity-induced mortality during their 50+ km trip to the sea.





Similar content being viewed by others
References
Ameyaw-Akumfi C (1989) Preliminary observations on seasonal movements of Cardisoma armatum Herklots, 1851. Crustaceana 57:223–224
Anger K (2003) Salinity as a key parameter in the larval biology of decapod crustaceans. Invertebr Reprod Dev 43:29–45
Anger K, Harms J, Montu M, Bakker C (1990) Effects of salinity on the larval development of a semiterrestrial tropical crab, Sesarma angustipes (Decapoda: Grapsidae). Mar Ecol Prog Ser 62:89–94
Anger K, Spivak E, Bas C, Ismael D, Luppi T (1994) Hatching rhythms and dispersion of decapod crustacean larvae in a brackish coastal lagoon in Argentina. Helgol Meeresunters 48:445–466
Anger K., Spivak E, Luppi T (1998) Effects of reduced salinities on development and bioenergetics of early larval shore crab, Carcinus maenas. J Exp Mar Biol Ecol 220:287–304
Behum ME, Brodie RJ, Staton JL (2005) Distribution of juvenile Uca pugnax and Uca pugilator across habitats in a South Carolina estuary, assessed by molecular techniques. Mar Ecol Prog Ser 288:211–220
Brodie RJ, Behum ME, Monroe E, Glenn N, Staton JL (2005) Recruitment to adult habitats following marine planktonic development in the fiddler crabs, Uca pugilator, U. pugnax, and U. minax. Mar Biol 147:105–111
Charmantier G, Gimenez L, Charmantier-Daures M (2001) Ontogeny of osmoregulation in crustaceans: the embryonic phase. Am Zool 41:1078–1089
Charmantier G, Gimenez L, Charmantier-Daures M, Anger K (2002) Ontogeny of osmoregulation, physiological plasticity and larval export strategy in the grapsid crab Chasmagnathys granulata (Crustacea, Decapoda). Mar Ecol Prog Ser 229:185–194
Christy JH (1982) Adaptive significance of semilunar cycles of larval release in fiddler crabs (Genus Uca): test of an hypothesis. Biol Bull 163:251–263
Christy JH, Stancyk SE (1982) Timing of larval production and flux of invertebrate larvae in a well-mixed estuary. In: Kennedy VS (ed) Estuarine comparisons. Academic, New York, pp 489–503
Crane J (1975) Fiddler crabs of the world. Princeton University Press, Princeton
Crisp DJ (1974) Factors influencing the settlement of marine invertebrate larvae. In: Grant PT, Mackie AM (eds) Chemoreceptors in marine organisms, Academic, London, pp 177–277
Crisp DJ (1976) Settlement responses in marine organisms. In: Newell RC (ed) Adaptation to environment: essays on the physiology of marine animals. Butterworths, London, pp 83–124
DeCoursey PJ (ed) (1979) Egg-hatching rhythms in three species of fiddler crabs. Pergamon Press, New York
DeCoursey PJ (ed) (1983) Biological timing. Academic, New York
De Vries MC, Tankersley RA, Forward RBJ, Kirby-Smith WW, Luettich RAJ (1994) Abundance of estuarine crab larvae is associated with tidal hydrologic variables. Mar Biol 118:403–413
De Wilde PAWJ (1973) On the ecology of Coenobita clypeatus in Curacao with reference to reproduction, water economy, and osmoregulation in terrestrial hermit crabs. Stud Fauna Curacao 44:1–138
Epifanio CE, Little KT, Rowe PM (1988) Dispersal and recruitment of fiddler crab larvae in the Delaware river estuary. Mar Ecol Prog Ser 43:181–188
Felder DL, Staton JL (1994) Genetic differentiation in trans-Floridian species complexes of Sesarma and Uca (Decapoda: Brachyura). J Crustac Biol 14:191–209
Felder JM, Felder DL, Hand SC (1986) Ontogeny of osmoregulation in the estuarine ghost shrimp Callianassa jamaicense var. Louisianensis Schmitt (Decapoda, Thalassinidea). J Exp Mar Biol Ecol 99:91–105
Finney DJ (1947) Probit analysis. Cambridge University Press, Cambridge
Forward RBJ (1987) Larval release rhythms of decapod crustaceans: an overview. Bull Mar Sci 41:165–176
Forward RBJ, Rittschof D (1994) Photoresponses of crab larvae in offshore and estuarine waters: implications for transport. J Exp Mar Biol Ecol 182:183–192
Gimenez L (2002) Effects of prehatching salinity and inital larval biomass on survival and duration of development in the zoea 1 of the estuarine crab, Chasmagnathus granulata, under nutritional stress. J Exp Mar Biol Ecol 270:93–110
Gimenez L, Torres G (2002) Larval growth in the estuarine crab Chasmagnathus granulata: the importance of salinity experienced during embryonic development, and the initial larval biomass. Mar Biol 141:877–885
Hare MP, Guenther C, Fagan WF (2005) Nonrandom larval dispersal can steepen marine clines. Evolution 59:2509–2517
Herborg LM, Rushton SP, Clare AS, Bentley MG (2003) Spread of the Chinese mitten crab (Eriocheir sinensis H. Milne Edwards) in Continental Europe: analysis of a historical data set. Hydrobiologia 503:21–28
Hill AE (1991a) A mechanism for horizontal zooplankton transport by vertical migration in tidal currents. Mar Biol 111:485–492
Hill AE (1991b) Advection-diffusion-mortality solutions for investigating pelagic larval dispersal. Mar Ecol Prog Ser 70:117–128
Le Conte J (1855) On a new species of Gelasimus. Proc Acad Nat Sci Philad 7:402–403
Morgan SG (1987) Adaptive significance of hatching rhythms and dispersal patterns of estuarine crab larvae: avoidance of physiological stress by larval export? J Exp Mar Biol Ecol 113:71–78
Morgan SG, Christy JH (1995) Adaptive significance of the timing of larval release by crabs. Am Nat 145:457–479
Morgan SG, Christy JH (1997) Planktivorous fishes as selective agents for reproductive synchrony. J Exp Mar Biol Ecol 209:89–101
O’Connor NJ, Epifanio CE (1985) The effect of salinity on the dispersal and recruitment of fiddler crab larvae. J Crustac Biol 5:137–145
Saigusa M, Hidaka T (1978) Semilunar rhythm in the zoea-release activity of the land crabs Sesarma. Oecologia 37:163–176
Sandifer PA (1975) The role of pelagic larvae in recruitment to populations of adult decapod crustaceans in the York River estuary and adjacent lower Chesapeake Bay, Virginia. Estuar Coastal Mar Sci 3:269–279
Tankersley RA, Wieber MG, Sigala MA, Kachurak KA (1998) Migratory behavior of ovigerous blue crabs, Callinectes sapidus: evidence for selective tidal stream transport. Biol Bull 195:168–173
Taylor HH, Seneviratna D (2005) Ontogeny of salinity tolerance and hyper-osmoregulation by embryos of the intertidal crabs Hemigrapsus edwardsii and Hemigrapsus crenulatus (Decapoda, Grapsidae): survival of acute hyposaline exposure. Comp Biochem Physiol Part A 140:495–505
Thorson G (1950) Reproductive and larval ecology of marine bottom invertebrates. Biol Rev 25:1–45
Tilburg CE, Reager JT, Whitney MM (2005) The physics of blue crab larval recruitment in Delaware Bay: a model study. J Mar Res 63:471–495
Young CM, Chia FS (1987) Abundance and distribution of pelagic larvae as influenced by predation, behavior and hydrographic factors. In: Giese C, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates. Blackwell Scientific and The Boxwood Press, Palo Alto and Pacific Grove, pp 385–463
Acknowledgments
We thank the staff at the Baruch Marine field Laboratory, especially Paul Kenny, for valuable assistance with field logistics. We also thank the West’s for allowing us to establish a field site at the Bates Hill Plantation. The Geoprocessing laboratory at Mount Holyoke College assisted us with our field site map. We thank Alan Kohn and two anonymous reviewers for valuable comments and suggestions on drafts of this work. This work was supported by the National Science foundation (NSF IBN-0237484) and the Office of Naval Research (N00014-02-1-0972). This is manuscript no. 1455 for the Baruch Marine Field Laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle.
Rights and permissions
About this article
Cite this article
Brodie, R.J., Styles, R., Borgianini, S. et al. Larval mortality during export to the sea in the fiddler crab Uca minax . Mar Biol 152, 1283–1291 (2007). https://doi.org/10.1007/s00227-007-0777-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0777-y