Abstract
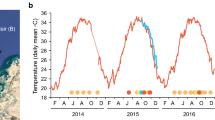
Rates of progression and transmission of black band disease (BBD) on the staghorn coral, Acropora muricata, were compared between months for seasonal in situ studies and between temperature treatments in experimental aquaria manipulations at Lizard Island on the Great Barrier Reef (GBR). In situ field experiments demonstrated that BBD progressed along branches approximately twice as fast (1.7–2.4 times) during the austral summer month of January (0.99 ± 0.04 cm/day) than in the cooler months of July (0.58 ± 0.04 cm/day) and May (0.41 ± 0.07 cm/day). Transmission of BBD between colonies was also accelerated in warmer months, with signs of infection becoming visible 1.2 days earlier in January compared to May. The greater seawater temperatures by ∼2 to 3°C and light intensities by up to 650 μE/m2/s in January, suggest that rates of progression and transmission of BBD are linked to one or both of these parameters. Manipulative experiments in summer provide corroborative evidence that elevated temperatures increase rates of BBD progression, with the disease progressing 1.3 times more rapidly in the 32°C elevated temperature treatment than in the 30°C ambient treatment (1.17 ± 0.06 cm/day versus 0.92 ± 0.07 cm/day; F2,6 = 7.66, P = 0.022). In contrast, although a trend for greatest BBD progression was measured in elevated temperature treatments of 29°C (0.46 ± 0.07 cm/day) and 31°C (0.52 ± 0.06 cm/day) in winter, these rates did not differ significantly (F3,7 = 1.72, P = 0.249) from those measured for the ambient 27°C treatment (0.37 ± 0.06 cm/day) or the field controls (0.41 ± 0.09 cm/day). The lower rates of BBD progression in the 31°C winter treatment (0.52 ± 0.06 cm/day) than in the 30°C (0.92 ± 0.07 cm/day) summer treatment, may have been a response to 28-fold decreased light irradiance in the former, suggesting that high irradiance in combination with elevated temperatures may promote progression of BBD. Results from this study indicate that the impact of elevated temperature on BBD progression is complex with a combination of environmental factors including temperature and light playing key roles in progression and transmission of the disease.




Similar content being viewed by others
References
Acosta A (2001) Disease in Zoanthids: dynamics in space and time. Hydrobiologia 460:113–130
Antonius A (1985) Coral diseases in the Indo-Pacific: a first record. PSZNI Mar Ecol 6:197–218
Antonius A, Riegl B (1997) A possible link between coral disease and a corallivorous snail (Drupella cornus) outbreak in the Read Sea. Atoll Res Bull 447:2–9
Aronson RB, Precht WF (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38
Banin E, Israely T, Fine M, Loya Y, Rosenberg E (2001) Role of endosymbiotic zooxanthellae and coral mucus in the adhesion of the coral-bleaching pathogen Vibrio shiloi to its host. FEMS Microbiol Lett 199:33–37
Bebout BM, Garcia-Pichel F (1995) UV-B induced vertical migrations of cyanobacteria in a microbial mat. Appl Environ Microbiol 61:4215–4222
Ben-Haim Y, Rosenberg E (2002) A novel sp. pathogen of the coral Pocillopora damicornis. Mar Biol 141:47–55
Berkelmans R, Oliver JK (1999) Large-scale bleaching of corals on the Great Barrier Reef. Coral Reefs 18:55–60
Berkelmans R, Willis BL (1999) Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18:219–228
Bruckner AW, Bruckner RJ (1997) The persistence of black band disease in Jamaica: impact on community structure. In: Proceedings of 8th International Coral Reef Symposium, vol 1, pp 601–606
Bruno JF, Petes LE, Harvell CD, Hettinger A (2003) Nutrient enrichment can increase the severity of coral diseases. Ecol Lett 6:1056–1061
Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, Sweatman H, Melendy AM (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol (in press)
Carlton RG, Richardson LL (1995) Oxygen and sulfide dynamics in a horizontally migrating cyanobacterial mat: Black band disease or corals. FEMS Microbiol Ecol 18:155–162
Castenholz RW (1982) Motility and taxes. In: Carr NG, Whitton BA (eds) The biology of cyanobacteria. University of California Press, Berkeley, pp 413–439
Coles SL, Jokiel PL (1978) Synergistic effects of temperature, salinity, and light on the hermatypic coral Montipora verrucosa. Mar Biol 49:187–195
Cooney RP, Pantos O, Le Tissier MDA, Barer MR, O’Donnell AG, Bythell JC (2002) Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environ Microbiol 4:401–413
Dinsdale E (1994) Coral black band disease: susceptibility, prevalence, community level influences and histopathology. Masters qualifying project. James Cook University, Australia
Ducklow HW, Mitchell R (1979) Observations of naturally and artificially diseased tropical corals: a scanning electron microscope study. Microb Ecol 5:215–223
Frias-Lopez J, Zerkle JAL, Bonheyo GT, Fouke BW (2002) Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Appl Environ Microbiol 68:2214–2228
Frias-Lopez J, Bonheyo GT, Jin Q, Fouke BW (2003) Cyanobacteria associated with coral black band disease in Caribbean and Indo-Pacific reefs. Appl Environ Microbiol 69:2409–2413
Garrett P, Ducklow P (1975) Coral disease in Bermuda. Nature 253:349–350
Griffith JK (1994) Predation on soft corals (Octocorallia: Alcyonacea) on the Great Barrier Reef, Australia. Aust J Mar Freshw Res 45:1281–1284
Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus ADME, Overstreet RM, Porter JW, Smith GW, Vasta GR (1999) Emerging marine disease—climate links and anthropogenic factors. Science 285:1505–1510
Harvell CD, Mitchell C, Ward J et al (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162
Hayes ML, Bonaventura J, Mitchell TP, Prospero JM, Smith EA, Dolah FV, Barber RT (2001) How are climate and marine biological outbreaks functionally linked? Hydrobiologia 460:213–220
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts and the resilience of coral reefs. Science 301:929–933
Jokiel PL, York RH (1982) Solar ultraviolet photobiology of the reef coral Pocillopora damicornis and symbiotic zooxanthellae. Bull Mar Sci 32:301–315
Karl TR, Trenberth KE (2003) Modern global climate change. Science 302:1719–1723
Kuta KG, Richardson LL (1996) Abundance and distribution of black band disease on coral reefs in the northern Florida Keys. Coral Reefs 15:219–223
Kuta KG, Richardson LL (2002) Ecological aspects of black band disease of corals: relationships between disease incidence and environmental factors. Coral Reefs 21:393–398
Lesser MP (2000) Depth-dependent photoacclimatization to solar ultraviolet radiation in the Caribbean coral Montastraea faveolata. Mar Ecol Prog Ser 192:137–151
McCallum H, Harvell CD, Dobson A (2003) Rates of spread of marine pathogens. Ecol Lett 6:1062–1067
Neudecker S (1979) Effects of grazing and browsing fishes on the zonation of corals in Guam. Ecology 60:666–672
Page C, Wills B (2006) Distribution, host range and large-scale spatial variability in black band disease prevalence on the Great Barrier Reef, Australia. Dis Aquat Organ 69:41–51
Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, Santavy DL, Smith GW (2002) The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Nat Acad Sci 99:8725–8730
Peters EC (1997) Diseases of coral-reef organisms. In: Birkeland C (ed) Life and death of coral reefs. Chapman and Hall, New York, pp 114–139
Porter JW, Tougas JI (2001) Reef ecosystems: threats to their biodiversity. In: Encyclopedia of biodiversity, vol 5. Academic, New York, pp 73–91
Porter JW, Dustan P, Jaap WC, Patterson KL, Kosmynin V, Meier OW, Patterson ME, Parsons M (2001) Patterns of spread of coral disease in the Florida Keys. Hydrobiologia 460:1–24
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Ramos-Flores T (1983) Lower marine fungus associated with black line disease in star corals (Montastrea annularis, E. & S.). Biol Bull 165:429–435
Richardson LL (1996) Horizontal and vertical migration patterns of Phormidium corallyticum and Beggiatoa spp. associated with black band disease of corals. Microb Ecol 32:323–335
Richardson LL, Kuta KG (2003) Ecological physiology of black band disease cyanobacterium Phormidium corallyticum. FEMS Microbiol Ecol 43:287–298
Rosenberg E, Ben-Haim Y (2002) Microbial diseases of corals and global warming. Environ Microbiol 4:318–326
Rützler K, Santavy DL (1983) The black band disease of Atlantic reef corals. I. Description of the cyanophyte pathogen. PSZNI Mar Ecol 4:301–319
Rützler K, Santavy DL, Antonius A (1983) The black band disease of Atlantic reef corals. III. Distribution, ecology and development. PSZNI Mar Ecol 4:329–358
Scheiner SM, Gurevitch J (2001) Design and anaysis of ecological experiments, 2nd edn. Oxford University Press, Oxford
Selig, ER, Harvell CD, Bruno JF, Willis BL, Page CA, Casey KS, Sweatman H (2006) Analyzing the relationship between ocean temperature anomalies and coral disease outbreaks at broad spatial scales. In: Phinney J, Hoegh-Guldberg O, Kleypas J, Skirving W, Strong A (eds) Coral reefs and climate change: science and management. AGU Coastal and Estuarine Series, vol 61
Sinha RP, Klisch M, Groniger A, Hader DP (2001) Responses of aquatic algae and cyanobacteria to solar UV-B. Plant Ecol 154:221–236
Stahl LJ (1995) Physiological ecology of cyanobacteria in microbial mats and other communities. New Phytol 131:1–32
Torren A, Landau L, Kushmaro A, Loya Y, Rosenberg E (1998) Effect of temperature on adhesion of Vibrio strain AK-1 to Oculina patagonica and on coral bleaching. Appl Environ Microbiol 64:1347–1384
Voss JD, Richardson LL (2006) Coral diseases near Lee Stocking Island, Bahamas: patterns and potential drivers. Dis Aquat Organ 69:33–40
Weil E (2004) Coral reef diseases in the wider Caribbean: status and prognosis. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, New York, pp 35–68
Willis BL, Page CA, Dinsdale EA (2004) Coral disease on the Great Barrier Reef. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, New York, pp 69–104
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall International, Inc., Upper Saddle River
Acknowledgments
We would like to thank N. Cantin, C. Page, M. Sussman, and the staff of Lizard Island Research Station for their assistance in field and laboratory studies. This research was supported by the Australian Research Council Discovery Program and the Global Environment Fund/World Bank Targeted Research and Capacity Building for Coral Reef Management program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.F. Humphrey.
Rights and permissions
About this article
Cite this article
Boyett, H.V., Bourne, D.G. & Willis, B.L. Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar Biol 151, 1711–1720 (2007). https://doi.org/10.1007/s00227-006-0603-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0603-y