Abstract
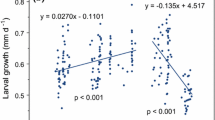
The influence of environmental variables on the planktonic growth, pelagic larval duration and settlement magnitude was examined for the coral reef surgeonfish Acanthurus chirurgus. Newly settled fish were collected daily from patch reefs in the San Blas Archipelago, Caribbean Panama for 3.5 years. Environmental influences on growth were examined at three different life history stages: from 0 to 6 days, 7 to 25 days and from 26 to 50 days after hatching. Larval growth was correlated, using multiple regression techniques, with a combination of factors including solar radiation, rainfall, and along-shore winds. Depending on the life history stage, these accounted for 13–38% of the variation in growth rates when all the months were included in the analyses. Correlations between environmental variables and growth also varied among seasons and were stronger in the dry than in the wet season. During the dry season solar radiation, rainfall and along-shore winds described 57%, 86% and 74% of the variability in growth between 0 and 6 days, 7 and 25 days and 26 and 50 days, respectively. During the wet season rainfall, along-shore winds and temperature only described 38% of the variability in early growth and 27% of growth just before settlement. No significant model was found to describe growth 7–25 days after hatching during the wet season. Rainfall, solar radiation and along-shore winds were negatively correlated with growth up to 25 days after hatching but positively correlated as larvae approached settlement at a mean age of 52 days. Over 65% of the variability in pelagic larval duration was accounted for by a regression model that included solar radiation and along-shore winds. When data sets from wet and dry seasons were analysed separately, along-shore winds accounted for 67% of the change in larval duration in the dry season, and solar radiation accounted for 23% of the variation in larval duration in the wet season. Only 22% of the variability in settlement intensity could be described by solar radiation and temperature, when all months of the year were included in the analysis. Solar radiation and rainfall were included in a regression model that accounted for 40% of the variation in numbers of fish settling during the dry season. This study suggests that the levels of solar radiation, along-shore winds and rainfall during the early larval life can have important effects on the growth, larval duration and consequently, the settlement magnitude of marine fishes. Results also highlight the need to account for seasonality and ontogeny in studies of environmental influences.




Similar content being viewed by others
References
Anderson JT (1988) A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. J Northwest Atl Fish Sci 8:55–66
Bailey KM, Houde ED (1989) Predation of eggs and larvae of marine fishes and the recruitment problem. Adv Mar Biol 25:1–83
Barnett V, Lewis T (1994) Outliers in statistical data. Wiley, England
Bergenius MAJ (1998) The influence of larval growth and stage duration on settlement variability in a coral reef fish. Honours thesis, Department of Marine Biology, James Cook University, Townsville
Bergenius MAJ, Meekan MG, Robertson DR, McCormick MI (2002) Larval growth predicts the recruitment success of a coral reef fish. Oecologia 131:521–525
Boeuf G, Le Bail PY (1999) Does light have an influence on fish growth? Aquaculture 177:129–152
Campana SE, Hurley PCF (1989) An age- and temperature-mediated growth model for cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) larvae in the Gulf of Maine. Can J Fish Aquat Sci 46:603–613
Chambers RC, Leggett WC (1987) Size and age at metamorphosis in marine fishes: an analysis of laboratory-reared winter flounder (Pseudopleuronectes americanus) with a review of variation in other species. Can J Fish Aquat Sci 44:1936–1947
Chambers RC, Miller TJ (1995) Evaluating fish growth by means of otolith increment analysis: special properties of individual level longitudinal data. pp 155-176 In: Secor DH, Dean JM, Camapana SE (eds) Recent developments in fish otolith research. University of South Carolina Press, South Carolina
Cubit JD, Caffey HM, Thompson RC, Windsor DM (1989) Meteorology and hydrography of a shoaling reef flat on the Caribbean coast of Panama. Coral Reefs 8:59–66
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv Mar Biol 26:250–293
Cushing DH (1995) Population production and regulation in the sea: a fisheries perspective. Cambridge University Press, Cambridge
Davies CA (2002) Statistical methods for the analysis of repeated measurements. Springer, Berlin Heidelberg New York
D’Croz L, Robertson DR, Martinez JA (1999) Cross-shelf distribution of nutrients, plankton, and fish larvae in the San Blas Archipelago, Caribb Panama Rev Biol Trop 47:203–215
Doherty PJ (1991) Spatial and temporal patterns in recruitment. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, San Diego, pp 261–293
Doherty PJ, Williams DMcB (1988) The replenishment of coral reef fish populations. Oceanogr Mar Biol Annu Rev 26:487–551
Dower JF, Miller TJ, Leggett WC (1997) The role of microscale turbulence in the feeding ecology of larval fish. Adv Mar Biol 31:170–220
Draper NR, Smith H (1981) Applied regression analysis. 2nd edn. Wiley, New York
Fortier L, Harris RP (1989) Optimal foraging and density-dependent competition in marine fish larvae. Mar Ecol Prog Ser 51:19–33
Fortier L, Gilbert M, Ponton D, Ingram G, Robinou B, Legendre L (1996) Impact of freshwater on a subarctic coastal ecosystem under seasonal ice (south-eastern Hudson Bay, Canada). III. Feeding success of marine fish larvae. J Mar Syst 7:251–265
Frank KT, Leggett WC (1981) Prediction of egg development and mortality rates in Capelin (Mallotus villosus) from meteorological, hydrographic, and biological factors. Can J Fish Aquat Sci 38:1327–1338
Fukahara O (1990) Effects of temperature on yolk utilisation, initial growth, and behaviour of unfed marine fish-larvae. Mar Biol 106:169–174
Gallego A, Heath MR, McKenzie E, Cargill LH (1996) Environmentally induced short-term variability in the growth rates of larval herring. Mar Ecol Prog Ser 137:11–23
Green BS, Fisher R (2004) Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J Exp Mar Biol Ecol 299:115–132
Heath MR (1992) Field investigations of the early life stages of marine fish. Adv Mar Biol 28:1–228
Heath MR, Henderson EW, Baird DL (1988) Vertical distribution of herring larvae in relation to physical mixing and illumination. Mar Ecol Prog Ser 47:211–228
Hjort J (1914) Fluctuations in the great fisheries of northern Europe reviewed in the light of biological research. Rapp PV Reun Cons Perm Int Explor Mer 20:1–128
Houde ED (1987) Fish early life dynamics and recruitment variability. Am Fish Soc Symp 2:17–29
Kouwenberg JHM, Browman HI, Runge JA, Cullen JJ, Davis RF, St-Pierre J-F (1999) Biological weighting of ultraviolet (280–400 nm) induced mortality in marine zooplankton and fish. II. Calanus finmarchicus (Copepoda) eggs. Mar Biol 134:269–284
Lesser MP, Farrell JH, Walker CW (2001) Oxidative stress, DNA damage and p53 expression in the larvae of Atlantic cod (Gadus morhua) exposed to ultraviolet (290–400 nm) radiation. J Exp Biol 204:157–164
McCormick MI, Hoey AS (2004) Larval growth history determines juvenile growth and survival in a tropical marine fish. Oikos 106:225–242
Meekan MG, Carleton JH, McKinnon AD, Flynn K, Furnas M.(2003) What determines the growth of tropical reef fish larvae in the plankton: Food or temperature? Mar Ecol Prog Ser 256:193–204
Polo A, Yufera M, Pascual E (1991) Effects of temperature on egg and larval development of Sparus auratus L. Aquaculture (Amsterdam) 92:367–375
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, UK
Randall JE (1961) A contribution to the biology of the convict surgeonfish of the Hawaiian Islands, Acanthurus triostegus. Pac Sci 15:215–272
Robertson DR (1992) Patterns of lunar settlement and early recruitment in Caribbean reef fishes at Panama. Mar Biol 114:527–537
Robertson DR, Swearer SE, Kaufmann K, Brothers EB (1999) Settlement vs. environmental dynamics in a pelagic-swimming reef fish at Caribbean Panama. Ecol Monogr 69:195–218
SAS (1987) SAS statistical guide. Version 6. Edition SAS Institute, Cary, N.C.
Searcy SP, Sponaugle S (2001) Selective mortality during the larval-juvenile transition in two coral reef fishes. Ecology 82:2452–2470
Suthers IM, Sundby S (1993) Dispersal and growth of pelagic juvenile Arcto-Norwegian cod (Gadus morhua), inferred from otolith microstructure and water temperature. ICES J Mar Sci 50:261–270
Thresher RE, Colin PL, Bell LJ (1989) Planktonic duration, distribution and population structure of Western and Central Pacific damselfishes (Pomacentridae). Copeia 1989:420–434
Utne-Palm AC, Stiansen JE (2002) Effect of larval ontogeny, turbulence and light on prey attack rate and swimming activity in herring larvae. J Exp Mar Biol Ecol 268:147–170
Vigliola L, Meekan MG (2002) Size at hatching and planktonic growth determines post-settlement survivorship of a coral reef fish. Oecologia 131: 89–93
Wellington GM, Victor BC (1989) Planktonic larval duration of one hundred species of Pacific and Atlantic damselfishes (Pomacentridae). Mar Biol 101:557–567
Wilson DT, McCormick MI (1999) Microstructure of settlement marks in the otoliths of tropical reef fish. Mar Biol 134:29–41
Wilson DT, Meekan MG (2002) Growth-related advantaged for survival to the point of replenishment in the coral reef fish Stegastes partitus (Pomacentridae). Mar Ecol Prog Ser 231:247–260
Zagarese HE, Williamson CE (2001) The implications of solar UV radiation exposure for fish and fisheries. Fish Fish 2:250–260
Acknowledgements
Statistical advice was gratefully received from D. Ryan, D. Donald and D. Coomans. We are grateful to B. Green and two anonymous reviewers who commented on the paper. Environmental data were provided by the Smithsonian Tropical Research Institute’s Marine Environmental Science Program. Logistic support was provided by James Cook University and the Australian Institute of Marine Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Bergenius, M.A.J., McCormick, M.I., Meekan, M.G. et al. Environmental influences on larval duration, growth and magnitude of settlement of a coral reef fish. Marine Biology 147, 291–300 (2005). https://doi.org/10.1007/s00227-005-1575-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-1575-z