Abstract
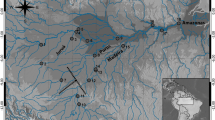
White shrimp (Litopenaeus vannamei) population genetic structure from the eastern Pacific was determined by restriction fragment length polymorphism analysis of the mitochondrial DNA control region. Four localities were surveyed with four endonucleases (Alu I, Taq I, Spe I, Ssp I) yielding 48 composite haplotypes. White shrimp showed high average within-locality haplotype (0.823) and nucleotide (5.41%) diversities and also high average nucleotide divergence between all pairs of localities (0.73%). A mismatch analysis of pairwise differences between haplotypes indicated that white shrimp does not fit the sudden population expansion model. An analysis of molecular variance showed significant geographic variation in the frequencies of haplotypes (ΦST=0.1382, P<0.0001). Population differentiation may be maintained by a combination of physical, oceanographic, and biological factors acting as barriers to gene flow among localities. Because of its high polymorphism, the control region might be useful as a genetic marker for monitoring genetic diversity in aquaculture stocks.


Similar content being viewed by others
References
Aubert H, Lightner DV (2000) Identification of genetic populations of the Pacific blue shrimp Penaeus stylirostris of the Gulf of California, Mexico. Mar Biol 137:875–885
Avise JC (1994) Molecular markers, natural history and evolution. Chapman & Hall, New York
Benzie JAH (2000) Population genetic structure in penaeid prawns. Aquacult Res 30:95–119
Castro JA, Picornell A, Ramon M (1998) Mitochondrial DNA: a tool for populational genetics studies. Int Microbiol 1:327–332
Chappell J (2002) Sea level changes forced ice breakouts in the last Glacial cycle: new results from coral terraces. Quaternary Sci Rev 21:1229–1240
Chakraborty R (1990) Mitochondrial DNA polymorphism reveals hidden heterogeneity within some Asian populations. Am J Hum Genet 47:87–94
Chu KH, Li CP, Tam YK, Lavery S (2003) Application of mitochondrial control region in population genetic studies of the shrimp Penaeus. Mol Ecol Notes 3:120–122
De la Rosa-Velez J, Escobar R, Correa F, Félix E (1999) High allozyme variation and genetic similarity of two populations of commercial penaeids, Penaeus brevirostris (Kingsley) and P. vannamei (Boone), from the Gulf of California. J R Stat Soc 30:459–463
Dore I, Frimodt C (1987) An illustrated guide to shrimp of the world. Osprey Books, Huntington
Duda TF Jr, Palumbi SR (1999) Population structure of the black tiger prawn, Penaeus monodon, among western Indian Ocean and western Pacific populations. Mar Biol 134:705–710
Excoffier L, Smouse P, Quattro J (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Felsenstein J (1993) Phylip (Phylogeny Inference Package), version 3.6c. Distributed by the author. Department of Genetics, University of Washington, Seattle
Féral JP (2002) How useful are the genetic markers in attempts to understand and manage marine biodiversity? J Exp Mar Biol Ecol 268:121–145
Garcia DK, Faggart MA, Rhoades L, Alcivar-Warren AA (1994) Genetic diversity of cultured Penaeus vannamei shrimp using three molecular genetic techniques. Mol Mar Biol Biotech 5:270–280
Garcia-Machado E, Robainas A, Espinosa G, Oliva M, Páez J, Verdecia N, Monnerot M (2001) Allozyme and mitochondrial DNA variation in Cuban populations of the shrimp Farfantepenaeus notialis (Crustacea: Decapoda). Mar Biol 138:701–707
Graves JE, McDowell JR (1994) Genetic analysis of striped marlin (Tetrapturus audax) population structure in the Pacific Ocean. Can J Fish Aquat Sci 51:1762–1768
Harrison RG (1989) Animal mitochondrial DNA as a genetic marker in population and evolutionary biology. Tree 1:6–11
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276
Hoolihan JP, Premanandh J, D’Aloia-Palmieri MA, Benzie JAH (2004) Intraspecific phylogeographic isolation of Arabian Gulf silfish Istiophorus platypterus inferred from mitochondrial DNA. Mar Biol 145:465–475
Kimura M, Crow JF (1964) The number of alleles that can be maintained in a finite population. Genetics 49:725–738
Klinbunga S, Penman DJ, McAndrew BJ, Tassanakajon A, Jarayabhand P (1998) Genetic variation, population differentiation, gene flow of the giant tiger shrimp (Penaeus monodon) inferred from mtDNA-RFLP data. In: Flegel TW (ed) Advances in shrimp biotechnology. National Center for Genetic Engineering and Biotechnology, Bangkok
Klinbunga S, Penman DJ, McAndrew BJ, Tassanakajon A (1999) Mitochondrial DNA diversity in three populations of the Giant Tiger Shrimp Penaeus monodon. Mar Biotechnol 1:113–121
Klinbunga S, Siludjai D, Wudthijinda W, Tassanakajon A, Jarayabhand P, Menasveta P (2001) Genetic heterogeneity of Giant Tiger Shrimp (Penaeus monodon) in Thailand revealed by RAPD and mitochondrial DNA RFLP analyses. Mar Biotechnol 3:428–438
Lavery S, Morit C, Fielder DR (1996) Indo-Pacific population structure and evolutionary history of the coconut crab Birgus latro. Mol Ecol 5:557–570
Lunt DH, Lawrence E, Whipple E, Hyman BC (1998) Mitochondrial DNA variable number tandem repeats (VNTRs): utility and problems in molecular ecology. Mol Ecol 7:1441–1455
Martínez-Córdova LR, Campaña-Torres A (1999) Especies de peneidos actuales y potenciales para el cultivo. In: Martinez LR (ed) Cultivo de camarones Peneidos: principios y prácticas. AGT, S. A. México, pp 23–38
McElroy DM, Moran P, Bermingham E, Kornfield I (1991) REAP: an integrated environment for the manipulation and phylogenetic analysis of restriction data. J Hered 83:157–158
McMillen-Jackson AL, Bert TM (2003) Disparate patterns of population genetic structure and population history in two sympatric penaeid shrimp species (Farfantepenaeus aztecus and Litopenaeus setiferus) in the eastern Unites States. Mol Ecol 12:2895–2905
McMillen-Jackson AL, Bert TM (2004) Genetic diversity in the mtDNA control region and population structure in the pink shrimp Farfantepenaeus duorarum. J Crust Biol 24(1):101–109
Meyer A (1994) DNA technology and phylogeny of fish. In: Beaumont AR (ed) Genetics and evolution of aquatic organisms. Chapman & Hall, London, pp 219–249
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nei M, Tajima F (1981) DNA polymorphism detectable by restriction endonucleases. Genetics 97:145–163
Raymond M, Rousset F (1995) GENEPOP (version 3.3): population genetics software for exact test and ecumenicism. J Hered 86:248–249
Rice WR (1989) Analyzing tables of statistical tests. Evolution 41:223–235
Rogers AR (1995) Genetic evidence for a Pleistocene population explosion. Evolution 49:608–615
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Ecol Evol 9:552–569
Rothlisberg PC (1982) Vertical migration and its effect on dispersal of penaeid shrimp postlarvae in the Gulf of Carpentaria, Australia. Fish Bull 80:541–554
Schneider S, Roessli D, Excoffier L (2000) Arlequin ver 2.000; a software for population genetics data analysis. Genetics and Biometry Laboratory, University of Geneva, Switzerland
Sunden SLF, Davis SK (1991) Evaluation of genetic variation in domestic population of Penaeus vannamei (Boone): a comparison with three natural populations. Aquaculture 97:131–142
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Thorpe JP, Solé-Cava AM, Watts PC (2000) Exploited marine invertebrates: genetics and fisheries. Hydrobiologia 420:165–184
Trasviña A, Lluch-Cota D, Filonov AE, Gallegos A (1999) Oceanografía y El Niño. In: Magaña VOR (ed) Los impactos de El Niño en México. UNAM, Mexico, pp 69–101
Valles-Jimenez R, Cruz P, Perez-Enriquez R (2004) Population genetic structure of Pacific white shrimp (Litopenaeus vannamei) from Mexico to Panama: microsatellite DNA variation. Mar Biotechnol 6:475–484
Van Hooft WF, Groen AF, Prins HHT (2002) Phylogeography of the African buffalo based on mitochondrial and Y-chromosomal loci: Pleistocene origin and population expansion of the Cape buffalo subspecies. Mol Ecol 11:267–279
Ward RD (2000) Genetics in fisheries management. Hydrobiologia 420:191–201
Weir BS, Cockerham CC (1984) Estimation F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wolfus GM, Garcia DK, Alcivar-Warren AA (1997) Application of the microsatellite technique for analyzing genetic diversity in shrimp breeding programs. Aquaculture 152:35–47
Zhang D, Hewitt G (2003) Nuclear DNA analyses in genetic studies of populations: practice, problems and prospects. Mol Ecol 12:563–584
Acknowledgements
Funding was provided by CONACYT (33496-V) and FOMIX Nayarit-CONACYT (2003-COI-9661) grants to R.P.E. The first author is a CONACYT graduate fellow (85938) and is grateful to the College of Marine Studies, University of Delaware, for the facilities provided during a research stay. Thanks to F. Garcia for his advice about the REAP program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. W. Sammarco, Chauvin
Rights and permissions
About this article
Cite this article
Valles-Jimenez, R., Gaffney, P.M. & Perez-Enriquez, R. RFLP analysis of the mtDNA control region in white shrimp (Litopenaeus vannamei) populations from the eastern Pacific. Marine Biology 148, 867–873 (2006). https://doi.org/10.1007/s00227-005-0122-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0122-2