Abstract
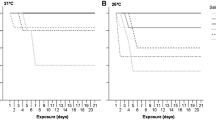
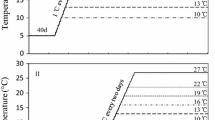
The successful invasion of non-indigeneous species depends on initial colonization as well as establishing a self-maintaining population. The invasive hydrozoan Moerisia lyonsi (Boulenger, 1908), possibly originating from low-salinity waters in the Black Sea and Middle East regions, has become established in low-salinity waters in several estuaries of North America, including Chesapeake Bay. The effects of temperature and salinity on mortality of M. lyonsi polyps were examined in the laboratory in February 2001 in the presence of abundant food. The polyps of M. lyonsi were directly transferred from 20°C and 10 salinity to one of 45 combinations of temperature (10–29°C) and salinity (1–40). Polyp mortality within 7 days occurred only in low-temperature treatments with salinities of 35–40. Surviving polyps reproduced asexually in salinities of 1–40 at 20–29°C, and in salinities of 1–25 at 15°C, but not in any salinities at 10°C. The greatest asexual reproduction rates, an index for population survival potential, occurred at salinities of 5–20. Survival and reproduction of M. lyonsi over such broad temperature and salinity ranges indicate that M. lyonsi may colonize and establish populations throughout the Chesapeake Bay; however, M. lyonsi medusae were reported only at salinities <9.3 there. This discrepancy may be due to the effects of predators. The scyphomedusan Chrysaora quinquecirrha (Desor, 1848), but not the ctenophore Mnemiopsis leidyi (A. Agassiz, 1865) consumed M. lyonsi medusae in laboratory experiments in August–September 2001. Populations of M. lyonsi do not appear to be limited by temperature and salinity conditions; however, their distribution in Chesapeake Bay may be restricted to low salinities not inhabited by predators.




Similar content being viewed by others
References
Arai MN (2001) Pelagic coelenterates and eutrophication: a review. Hydrobiologia 451:69–87
Boero F (1984) The ecology of marine hydroids and effects of environmental factors: a review. Mar Ecol 5:93–118
Caddy JF, Griffiths RC (1990) Recent trends in the fisheries and environment in the General Fisheries Council for the Mediterranean (GFCM) area. Gen Fish Counc Mediterr Stud Rev 63:43–71
Calder DR (1971) Hydroids and hydromedusae of southern Chesapeake Bay. Special papers in marine science no. 1, Virginia Institute of Marine Science
Calder DR (1976) The zonation of hydroids along salinity gradients in South Carolina estuaries. In: Mackie GO (ed) Coelenterate ecology and behavior. Plenum, New York, pp 165–174
Calder DR, Burrell VG Jr (1967) Occurrence of Moerisia lyonsi (Limnomedusae. Moerisiidae) in North America. Am Midl Nat 78:540–541
Calder DR, Burrell VG Jr (1969) Brackish water hydromedusa Maeotias inexpectata in North America. Nature 222:694–695
Cargo DG, Burnett JW (1982) Observations on the ultrastructure and defensive behavior of the cnidosac of Cratena pilata. Veliger 24:325–327
Cargo DG, Schultz LP (1966) Notes on the biology of the sea nettle, Chrysaora quinquecirrha, in Chesapeake Bay. Chesapeake Sci 7:95–100
Cargo DG, Schultz LP (1967) Further observations on the biology of the sea nettle and jellyfishes in the Chesapeake Bay. Chesapeake Sci 8:209–220
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of non-indigenous marine organisms. Science 261:78–82
Cervetto G, Gaudy R, Pagano M (1999) Influence of salinity on the distribution of Acartia tonsa (Copepoda, Calanoida). J Exp Mar Biol Ecol 239:33–45
Cones HN Jr, Haven DS (1969) Distribution of Chrysaora quinquecirrha in the York River. Chesapeake Sci 10:75–84
Cowan JH, Houde ED (1993) Relative predation potentials of scyphomedusae, ctenophores and planktivorous fish on ichthyoplankton in Chesapeake Bay. Mar Ecol Prog Ser 95:55–65
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139
Dumont HJ (1994) The distribution and ecology of the fresh- and brackish-water medusae of the world. Hydrobiologia 272:1–12
Feigenbaum D, Kelly M (1984) Changes in the lower Chesapeake Bay food chain in presence of the sea nettle Chrysaora quinquecirrha (Scyphomedusa). Mar Ecol Prog Ser 19:39–47
Folino NC (1993) Feeding and growth of the aeolid nudibranch Cuthona nana (Alder and Hancock, 1842). J Mollusc Stud 59:15–22
Ford MD, Costello JH, Heidelberg KB (1997) Swimming and feeding by the scyphomedusa Chrysaora quinquecirrha. Mar Biol 129:355–362
Hernroth L, Gröndahl F (1985) On the biology of Aurelia aurita (L.). 3. Predation by Coryphella verrucosa (Gastropoda, Opisthobranchia), a major factor regulating the development of Aurelia populations in the Gullmar Fjord, western Sweden. Ophelia 24:37–45
Khlebovich VV (1968) Some peculiar features of the hydrochemical regime and the fauna of mesohaline waters. Mar Biol 2:47–49
Khlebovich VV (1969) Aspects of animal evolution related to critical salinity and internal state. Mar Biol 2:338–345
Kideys AE (1994) Recent dramatic changes in the Black Sea ecosystem: the reason for the sharp decline in Turkish anchovy fisheries. J Mar Syst 5:171–181
Kinne O (ed) (1970) Temperature. Animals. Invertebrates. In: Marine ecology. A comprehensive integrated treatise on life in oceans and coastal waters. Wiley-Interscience, New York, pp 407–514
Kinne O (ed) (1971) Salinity. Animals. Invertebrates. In: Marine ecology. A comprehensive integrated treatise on life in oceans and coastal waters. Wiley-Interscience, New York, pp 821–995
Kovalev AV, Mazzocchi MG, Siokou-Frangou I, Kideys AE (2001) Zooplankton of the Black Sea and the eastern Mediterranean: similarities and dissimilarities. Mediterr Mar Sci 2:69–77
Larson RJ (1987) Trophic ecology of planktonic gelatinous predators in Saanich Inlet, British Columbia: diets and prey selection. J Plankton Res 9:811–820
Letunov V, Marfenin N (1980) Some characteristics of feeding behavior in winter colonies of Dynamena pumila under various temperature regimens (in Russian). Biol Nauk 6:51–55
Ma X, Purcell JE (2005) Temperature, salinity, and prey effects on polyp versus medusa bud production by the invasive hydrozoan Moerisia lyonsi. Mar Biol (in press). DOI 10.1007/s00227-004-1539-8
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Mackie GL (1991) Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 219:251–268
McDonald G, Nybakken J (1997) A worldwide review of the food of nudibranch mollusks. 1. Introduction and the suborder Arminacea. Veliger 40:157–159
McDonald G, Nybakken J (1999) A worldwide review of the food of nudibranch mollusks, part II. The suborder Dendronotacea. Veliger 42:62–66
Mills CE (2001) Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451:55–68
Mills CE, Rees JT (2000) New observations and corrections concerning the trio of invasive hydromedusae Maeotias marginata (=M. inexpectata), Blackfordia virginica, and Moerisia sp. in the San Francisco estuary. Sci Mar 64:151–155
Mills CE, Sommer F (1995) Invertebrate introductions in marine habitats: two species of hydromedusae (Cnidaria) native to the Black Sea, Maeotias inexspectata and Blackfordia virginica, invade San Francisco Bay. Mar Biol 122:279–288
OTA (U.S. Congress, Office of Technology Assessment) (1993) Harmful non-indigenous species in the United States. OTF-F-565, US Government Printing Office, Washington, D.C., USA
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: assessing the ecological effects of invaders. J Biol Invest 1:3–19
Petersen JE, Sanford LP, Kemp WM (1998) Coastal plankton responses to turbulent mixing in experimental ecosystems. Mar Ecol Prog Ser 171:23–41
Poirrier MA, Mulino MM (1977) The impact of the 1975 Bonnet Carre spillway opening on epifaunal invertebrates of Lake Pontchartrain. J Elisha Mitchell Sci Soc 93:11–17
Purcell JE (1992) Effects of predation by the scyphomedusan Chrysaora quinquecirrha on zooplankton populations in Chesapeake Bay, USA. Mar Ecol Prog Ser 87:65–76
Purcell JE (1997) Pelagic cnidarians and ctenophores as predators: selective predation, feeding rates, and effects on prey populations. Ann Inst Oceanogr Paris 73:125–137
Purcell JE, Cowan JH Jr (1995) Predation by the scyphomedusan Chrysaora quinquecirrha on Mnemiopsis leidyi ctenophores. Mar Ecol Prog Ser 129:63–70
Purcell JE, Decker MB (2005) Effects of climate during 1987–2000 on relative predation by ctenophores and scyphomedusae on copepods in Chesapeake Bay. Limnol Oceanogr 50:376–387
Purcell JE, Grover JJ (1990) Predation and food limitation as causes of mortality in larval herring at a spawning ground in British Columbia. Mar Ecol Prog Ser 59:55–61
Purcell JE, Nemazie DA, Dorsey SE, Houde ED, Gamble JC (1994a) Predation mortality of bay anchovy (Anchoa mitchilli) eggs and larvae due to scyphomedusae and ctenophores in Chesapeake Bay. Mar Ecol Prog Ser 114:47–58
Purcell JE, White JR, Roman MR (1994b) Predation by gelatinous zooplankton and resource limitation as potential controls of Acartia tonsa copepod populations in Chesapeake Bay. Limnol Oceanogr 39:263–278
Purcell JE, Båmstedt U, Båmstedt A (1999a) Prey, feeding rates, and asexual reproduction rates of the introduced oligohaline hydrozoan Moerisia lyonsi. Mar Biol 134:317–325
Purcell JE, Malej A, Benović A (1999b) Potential links of jellyfish to eutrophication and fisheries. Coast Estuar Stud 55:241–263
Purcell JE, White JR, Nemazie DA, Wright DA (1999c) Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Mar Ecol Prog Ser 180:187–196
Purcell JE, Graham WM, Dumont HJ (eds) (2001a) Jellyfish blooms: ecological and societal importance. Kluwer, Amsterdam
Purcell JE, Shiganova TA, Decker MB, Houde ED (2001b) The ctenophore Mnemiopsis in native and exotic habitats: U.S. estuaries versus the Black Sea basin. Hydrobiologia 451:145–176
Rees JT, Gershwin LA (2000) Non-indigenous hydromedusae in California’s upper San Francisco estuary: life cycles, distribution, and potential environmental impacts. Sci Mar 64[Suppl 1]:73–86
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am Zool 37:621–632
Sagasti A, Schaffner LC, Duffy JE (2000) Epifaunal communities thrive in an estuary with hypoxic episodes. Estuaries 23:474–487
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Sandifer PA, Smith TLJ, Calder DR (1974) Hydrozoans as pests in closed-system culture of larval decapod crustaceans. Aquaculture 4:55–59
Shiganova TA (1998) Invasion of ctenophore Mnemiopsis leidyi in the Black Sea and recent changes in its pelagic community structure. Fish Oceanogr[Globec Special Issue]:305–310
Simkina RG (1980) A quantitative feeding study of the colonies of Perigonimus megas (Hydroida, Bougainvillidae) (in Russian). Zool Zh 59:500–506
Smith LD, Wonham MJ, McCann LD, Ruiz GM, Hines AH, Carlton JT (1999) Invasion pressure and inoculant survival in a ballast-flooded estuary. J Biol Invest 1:67–87
Stokes ME, Davis CS, Koch GG (1991) Categorical data analysis using the SAS system, 2nd edn. SAS Institute and Wiley, Cary, N.C., USA
Travis J (1993) Invader threatens Black, Azov Seas. Science 262:1366–1367
Turpaeva E, Gal’perin M, Simkina R (1977) Food availability and energy flux in the matured colonies of the hydroid polyp Perigonimus megas Kinne (in Russian). Okeanologiya 17:1090–1101
Vinogradov M, Shushkina ELA, Bulgakova YuV, Serobaba II (1995) Consumption of zooplankton by the atenophore Mnemiopsis leydyi and pelagic fish in the Black Sea. Okeanologiya/Oceanology (Moscow) 35:569–573
Wonham MJ, Walton WC, Ruiz GM, Frese AM, Galil BS (2001) Going to the source: role of the invasion pathway in determining potential invaders. Mar Ecol Prog Ser 215:1–12
Wright DA, Purcell JE (1997) Effect of salinity on ionic shifts in mesohaline scyphomedusae, Chrysaora quinquecirrha. Biol Bull (Woods Hole) 192:332–339
Acknowledgements
This research was financially supported by the Horn Point Laboratory and the Multiscale Experimental Ecosystem Research Center (MEERC) at the University of Maryland Center for Environmental Science (EPA grant no. R819640). We thank R.W. Osman (the Academy of Natural Science Estuarine Research Center, at St. Leonard, Md.) for supplying settling plates with Moerisia lyonsi polyps, and T.J. Miller for suggestions on statistical analysis. We especially thank V.S. Kennedy for encouragement, advice, and editing on earlier versions of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Ma, X., Purcell, J.E. Effects of temperature, salinity, and predators on mortality of and colonization by the invasive hydrozoan Moerisia lyonsi. Marine Biology 147, 215–224 (2005). https://doi.org/10.1007/s00227-004-1538-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1538-9