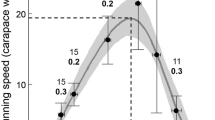
Abstract
Sand shrimp, Crangon septemspinosa Say, are important to the trophic dynamics of coastal systems in the northwestern Atlantic. To evaluate predatory impacts of sand shrimp, daily energy requirements (J ind.−1 day−1) were calculated for this species from laboratory estimates of energy losses due to routine (R R), active (R A), and feeding (R SDA) oxygen consumption rates (J ind.−1 h−1), coupled with measurements of diel motile activity. Shrimp used in this study were collected biweekly from the Niantic River, Connecticut (41°33′N; 72°19′W) during late spring and summer of 2000 and 2001. The rates of shrimp energy loss due to R R and R A increased exponentially with increasing temperature, with the magnitude of increase greater between 6°C and 10°C (Q 10=3.01) than between 10°C and 14°C (Q 10=2.85). Rates of R R doubled with a twofold increase in shrimp mass, and R SDA was 0.130 J h−1+R R, irrespective of shrimp body size. Shrimp motile activity was significantly greater during dark periods relative to light periods, indicating nocturnal behavior. Nocturnal activity also increased significantly at higher temperatures, and at 20°C shifted from a unimodal to a bimodal pattern. Laboratory estimates of daily metabolic expenditures (1.7–307.4 J ind.−1 day−1 for 0.05 and 1.5 g wet weight shrimp, respectively, between 0°C and 20°C) were combined with results from previous investigations to construct a bioenergetic model and make inferences regarding the trophic positioning of C. septemspinosa. Bioenergetic model estimates indicated that juvenile and adult shrimp could meet daily energy demands via opportunistic omnivory, selectively preying upon items of high energy content (e.g. invertebrate and fish tissue) and compensating for limited prey availability by ingesting readily accessible lower energy food (e.g. detritus and plant material).






Similar content being viewed by others
References
Berdalet E, Packard T, Lagacé B, Roy S, St.-Amand L, Gagné J-P (1995) CO2 production, O2 consumption and isocitrate dehydrogenase in the marine bacterium Vibrio natriegens. Aquat Microb Ecol 9:211–217
Bishop JM, Gosselink JG, Stone JH (1980) Oxygen consumption and hemolymph osmolality of brown shrimp, Penaeus aztecus. Fish Bull (Wash DC) 78:741–757
Branch GM, Newell RC (1978) A comparative study of metabolic energy expenditure in the limpets Patella cochlear, P. oculus and P. granularis. Mar Biol 49:351–361
Cockcroft AC, Wooldridge T (1985) The effects of mass, temperature and molting on the respiration of Macropetasma africanus Balss (Decapoda: Penaeoidea). Comp Biochem Physiol 81:143–148
Corey S (1987) Reproductive strategies and comparative fecundity of Crangon septemspinosa Say (Decapoda, Caridea). Crustaceana 52:25–28
Czekajewski J, Nennerfelt L, Kaczmarek H, Rabek JF (1994) Application of a new generation of computerized apparatus for the study of oxygen uptake and production of CO and CO2 during photo-(thermal) oxidation of polymers. Acta Polymerica 45:369–374
Dall W (1965) Studies on the physiology of a shrimp, Metapenaeus sp. (Crustacea: Decapoda: Penaeidae). IV. Carbohydrate metabolism. Aust J Mar Freshw Res 16:163–180
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59:433–463
Du Prez HH, Chen H-Y, Hsieh C-S (1992) Apparent specific dynamic action of food in the grass shrimp, Penaeus monodon Fabricius. Comp Biochem Physiol 103:173–178
Elliott JM, Davison W (1975) Energy equivalents of oxygen consumption in animal energetics. Oecologia 19:195–201
Fry FEJ (1957) The aquatic respiration of fish. In: Brown ME (ed) The physiology of fishes, vol I. Academic, New York, pp 1–63
Gibson RN, Yin MC, Robb L (1995) The behavioural basis of predator–prey size relationships between shrimp (Crangon crangon) and juvenile plaice (Pleuronectes platessa). J Mar Biol Assoc UK 75:337–349
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Haefner PA (1969) Temperature–salinity tolerance of the sand shrimp, Crangon septemspinosa Say. Physiol Zool 42:388–397
Haefner PA (1973) Length–weight relationship of the sand shrimp, Crangon septemspinosa. Chesapeake Sci 14:141–143
Haefner PA (1979) Comparative review of the biology of North Atlantic caridean shrimps (Crangon) with emphasis on C. septemspinosa. Bull Biol Soc Wash 3:1–40
Hagerman L (1976) The respiration during the molt cycle of Crangon vulgaris (Fabr), (Crustacea, Natantia). Ophelia 15:15–21
Jorde DG, Owen RB Jr (1990) Changes in caloric content of the amphipod Gammarus oceanicus along the coast of Maine. Can Field-Nat 104:303–304
Kurmaly K, Yule AB, Jone DA (1989) Effects of body size and temperature on the metabolic rate of Penaeus monodon. Mar Biol 103:25–30
Kutty MN, Murugapoopathy G, Krishnan TS (1974) Influence of salinity and temperature on the oxygen consumption in young juveniles of the Indian prawn Penaeus indicus. Mar Biol 11:125–131
Lankford TE, Targett TE (1997) Selective predation by juvenile weakfish: post-consumptive constraints on energy maximization and growth. Ecology 78:1049–1061
McFarland WN, Pickens PE (1965) The effects of season, temperature, and salinity on standard and active oxygen consumption of the grass shrimp Palaemonetes vulgaris (Say). Can J Zool 43:571–585
Miac J, Groth M, Wolowicz M (1997) Seasonal changes in the Mya arenaria (L.) population from inner Puck Bay. Oceanologia 39:177–195
Modlin RF (1980) The life cycle and recruitment of the sand shrimp, Crangon septemspinosa, in the Mystic River estuary, Connecticut. Estuaries 3:1–10
Nelson SG, Li HW, Knight AW (1977) Calorie, carbon and nitrogen metabolism of juvenile Macrobrachium rosenbergii (De Man) (Crustacea, Palaemonidae) with regard to trophic position. Comp Biochem Physiol 58:319–327
Nelson SG, Simmons MA, Knight AW (1979) Ammonia excretion by the benthic estuarine shrimp Crangon franciscorum (Crustacea: Crangonidae) in relation to diet. Mar Biol 54:25–31
Price KSJ (1962) Biology of the sand shrimp, Crangon septemspinosa, in the shore zone of the Delaware Bay region. Chesapeake Sci 3:244–255
Regnault M (1981) Respiration and ammonia excretion of the shrimp Crangon crangon L.: metabolic response to prolonged starvation. J Comp Physiol 141:549–555
Regnault M, Lagardère JP (1983) Effects of ambient noise on the metabolic level of Crangon crangon (Decapoda, Natantia). Mar Ecol Prog Ser 11:71–78
Ross LG, MicKinney RW (1988) Respiratory cycles in Oreochromis niloticus (L), measured using a six-channel microcomputer-operated respirometer. Comp Biochem Physiol A 89:637–643
Rudnick DT, Elmgren R, Frithsen JB (1985) Meiofaunal prominence and benthic seasonality in a coastal marine ecosystem. Oecologia 67:157–168
Subrahmanyam CB (1962) Oxygen consumption in relation to body weight and oxygen tension in the prawn Penaeus indicus (Milne Edwards). Proc Indian Acad Sci 55B:152–161
Subrahmanyam CB (1976) Tidal and diurnal rhythms of locomotory activity and oxygen consumption in the pink shrimp, Penaeus duorarum. Contrib Mar Sci 20:123–132
Szaniawska A (1983) Seasonal changes in energy content of Crangon crangon L. (Crustacea, Decapoda). Pol Arch Hydrobiol 30:45–56
Szaniawska A, Wolowicz M (1984) Seasonal changes of in oxygen consumption by Crangon crangon L. (Crustacea, Natantia) in the Gulf of Gdańsk. Oceanologia 19:117–126
Underwood AJ (1981) Techniques of analysis of variance in experimental marine biology and ecology. Oceanogr Mar Biol Annu Rev 19:513–605
Van Donk E, De Wilde PAWJ (1981) Oxygen consumption and motile activity of the brown shrimp Crangon crangon related to temperature and body size. Neth J Sea Res 15:4–64
Villarreal H, Ocampo L (1993) Effect of size and temperature on the oxygen consumption of the brown shrimp Penaeus californiensis (Holmes, 1900). Comp Biochem Physiol A 106:97–101
Villarreal H, Rivera JA (1993) Effect of temperature and salinity on the oxygen consumption of laboratory produced Penaeus californiensis postlarvae. Comp Biochem Physiol A 106:103–107
Von Ende CN (1993) Repeated-measures analysis: growth and other time-dependent measures. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 113–137
Vonk HJ (1960) Digestion and metabolism. In: Waterman TH (ed) The physiology of Crustacea. Academic, New York, pp 291–316
Weisse T, Rudstam LG (1989) Excretion and respiration rates of Neomysis integer (Mysidaceae): effects of temperature, sex and starvation. Hydrobiologia 178:253–258
Welch HE (1968) Relationships between assimilation efficiencies and growth efficiencies for aquatic consumers. Ecology 49:755–759
Whiteley NM, Robertson RF, Meagor J, El Haj AJ, Taylor EW (2001) Protein synthesis and specific dynamic action in crustaceans: effects of temperature. Comp Biochem Physiol 128:595–606
Wilcox JR (1972) Feeding habits of the sand shrimp, Crangon septemspinosa (Crustacea, Decapoda, Natantia). PhD thesis, University of Rhode Island, Providence
Wilcox JR, Jeffries HP (1973) Growth of the sand shrimp, Crangon septemspinosa, in Rhode Island. Chesapeake Sci 14:201–205
Wilcox JR, Jeffries HP (1974) Feeding habits of the sand shrimp Crangon septemspinosa. Biol Bull (Woods Hole) 146:424–434
Wolvenkamp HP, Waterman TH (1960) Respiration. In: Waterman TH (ed) The physiology of Crustacea. Academic, New York, pp 35–100
Acknowledgements
We are grateful to J.S. Collie, and two anonymous referees for scientific and editorial reviews, which greatly improved this manuscript. We also thank D.J. Danila for providing shrimp, and US EPA (AED) and M. Dickson for the allocation of laboratory space and equipment to conduct respiration studies. This research was partially funded by the Joshua MacMillan Graduate Fellowship in Fisheries Oceanography, Germaine and Francis Webb Graduate Fellowship in Oceanography, and URI/NOAA Cooperative Marine Education and Research (award no. NA17FE2312).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Taylor, D.L., Peck, M.A. Daily energy requirements and trophic positioning of the sand shrimp Crangon septemspinosa . Marine Biology 145, 167–177 (2004). https://doi.org/10.1007/s00227-004-1299-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1299-5