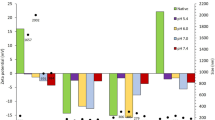
Abstract
Autosomal dominant osteopetrosis type 2 (ADO2) is a rare inherited bone disorder characterised by dense but brittle bones. It displays striking phenotypic variability, with the most severe symptoms, including blindness and bone marrow failure. Disease management largely relies on symptomatic treatment since there is no safe and effective treatment. Most ADO2 cases are caused by heterozygous loss-of-function mutations in the CLCN7 gene, which encodes an essential Cl−/H+ antiporter for proper bone resorption by osteoclasts. Thus, siRNA-mediated silencing of the mutant allele is a promising therapeutic approach, but targeting bone for first-in-human translation remains challenging. Here, we demonstrate the utility of silicon-stabilised hybrid lipid nanoparticles (sshLNPs) as a next-generation nucleic acid nanocarrier capable of delivering allele-specific siRNA to bone. Using a Clcn7G213R knock-in mouse model recapitulating one of the most common human ADO2 mutations and based on the 129S genetic background (which produces the most severe disease phenotype amongst current models), we show substantial knockdown of the mutant allele in femur when siRNA targeting the pathogenic variant is delivered by sshLNPs. We observed lower areal bone mineral density in femur and reduced trabecular thickness in femur and tibia, when siRNA-loaded sshLNPs were administered subcutaneously (representing the most relevant administration route for clinical adoption and patient adherence). Importantly, sshLNPs have improved stability over conventional LNPs and enable ‘post hoc loading’ for point-of-care formulation. The treatment was well tolerated, suggesting that sshLNP-enabled gene therapy might allow successful clinical translation of essential new treatments for ADO2 and potentially other rare genetic bone diseases.





Similar content being viewed by others
References
Capulli M, Maurizi A, Ventura L, Rucci N, Teti A (2015) Effective small interfering RNA therapy to treat CLCN7-dependent autosomal dominant osteopetrosis type 2. Mol Ther Nucleic Acids 4(9):e248. https://doi.org/10.1038/mtna.2015.21
Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, Johnson M, Kocisko M, Evans WE, Foroud T, Econs MJ (2003) Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res 18(8):1513–1518. https://doi.org/10.1359/jbmr.2003.18.8.1513
Waguespack SG, Hui SL, Dimeglio LA, Econs MJ (2007) Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metab 92(3):771–778. https://doi.org/10.1210/jc.2006-1986
Coudert AE, Del Fattore A, Baulard C, Olaso R, Schiltz C, Collet C, Teti A, de Vernejoul M-C (2014) Differentially expressed genes in autosomal dominant osteopetrosis type II osteoclasts reveal known and novel pathways for osteoclast biology. Lab Invest 94(3):275–285. https://doi.org/10.1038/labinvest.2013.140
Alam I, Gray AK, Chu K, Ichikawa S, Mohammad KS, Capannolo M, Capulli M, Maurizi A, Muraca M, Teti A, Econs MJ, Del Fattore A (2014) Generation of the first autosomal dominant osteopetrosis type II (ADO2) disease models. Bone 59:66–75. https://doi.org/10.1016/j.bone.2013.10.021
Henriksen K, Gram J, Schaller S, Dahl BH, Dziegiel MH, Bollerslev J, Karsdal MA (2004) Characterization of osteoclasts from patients harboring a G215R mutation in ClC-7 causing autosomal dominant osteopetrosis type II. Am J Pathol 164(5):1537–1545. https://doi.org/10.1016/s0002-9440(10)63712-1
Sobacchi C, Villa A, Schulz A, Kornak U (2022) CLCN7-related osteopetrosis. In: Adam MP, Mirzaa GM, Pagon RA et al (eds) GeneReviews. University of Washington, Seattle
Maurizi A, Capulli M, Patel R, Curle A, Rucci N, Teti A (2018) RNA interference therapy for autosomal dominant osteopetrosis type 2 towards the preclinical development. Bone 110:343–354. https://doi.org/10.1016/j.bone.2018.02.031
Cohen-Solal M, Collet C, Bizot P, Pavis C, Funck-Brentano T (2023) Osteopetrosis: the patient point of view and medical challenges. Bone 167:116635. https://doi.org/10.1016/j.bone.2022.116635
Wu CC, Econs MJ, DiMeglio LA, Insogna KL, Levine MA, Orchard PJ, Miller WP, Petryk A, Rush ET, Shoback DM, Ward LM, Polgreen LE (2017) Diagnosis and management of osteopetrosis: consensus guidelines from the osteopetrosis working group. J Clin Endocrinol Metab 102(9):3111–3123. https://doi.org/10.1210/jc.2017-01127
Chen Y, Zhou L, Guan X, Wen X, Yu J, Dou Y (2023) Case report: Gene mutations and clinical characteristics of four patients with osteopetrosis. Front Pediatr 11:1096770. https://doi.org/10.3389/fped.2023.1096770
Maurizi A (2022) Experimental therapies for osteopetrosis. Bone 165:116567. https://doi.org/10.1016/j.bone.2022.116567
Xiong Q, Zhang L, Ge W, Tang P (2016) The roles of interferons in osteoclasts and osteoclastogenesis. Joint Bone Spine 83(3):276–281. https://doi.org/10.1016/j.jbspin.2015.07.010
Alam I, Gray AK, Acton D, Gerard-O’Riley RL, Reilly AM, Econs MJ (2015) Interferon gamma, but not calcitriol improves the osteopetrotic phenotypes in ADO2 mice. J Bone Miner Res 30(11):2005–2013. https://doi.org/10.1002/jbmr.2545
Imel EA, Liu Z, Acton D, Coffman M, Gebregziabher N, Tong Y, Econs MJ (2019) Interferon gamma-1b does not increase markers of bone resorption in autosomal dominant osteopetrosis. J Bone Miner Res 34(8):1436–1445. https://doi.org/10.1002/jbmr.3715
Nguyen A, Miller WP, Gupta A, Lund TC, Schiferl D, Lam LSK, Arzumanyan Z, Orchard PJ, Polgreen LE (2022) Open-label pilot study of interferon gamma-1b in patients with non-infantile osteopetrosis. JBMR Plus 6(3):e10597. https://doi.org/10.1002/jbm4.10597
Miller VM, Xia H, Marrs GL, Gouvion CM, Lee G, Davidson BL, Paulson HL (2003) Allele-specific silencing of dominant disease genes. Proc Natl Acad Sci U S A 100(12):7195–7200. https://doi.org/10.1073/pnas.1231012100
Liu X (2016) Bone site-specific delivery of siRNA. J Biomed Res 30(4):264–271. https://doi.org/10.7555/JBR.30.20150110
Malcolm DW, Wang Y, Overby C, Newman M, Benoit DSW (2020) Delivery of RNAi-based therapeutics for bone regeneration. Curr Osteoporos Rep 18(3):312–324. https://doi.org/10.1007/s11914-020-00587-2
Saffie-Siebert RS, Torabi-Pour N, Ahmed N, inventors; SiSaf Limited, assignee. A delivery system comprising silicon-containing material. WIPO Patent Application WO 2020/193995 A1. March 30, 2020.
Saffie-Siebert RS, Ahmed M, Sutera F, inventors; SiSaf Limited, assignee. Compositions comprising doped silicon particles, and related methods. WIPO Patent Application WO 2023/002222 A1. July 22, 2022.
Friedrich M, Aigner A (2022) Therapeutic siRNA: state-of-the-art and future perspectives. BioDrugs 36(5):549–571. https://doi.org/10.1007/s40259-022-00549-3
Baran-Rachwalska P, Torabi-Pour N, Sutera FM, Ahmed M, Thomas K, Nesbit MA, Welsh M, Moore CBT, Saffie-Siebert SR (2020) Topical siRNA delivery to the cornea and anterior eye by hybrid silicon–lipid nanoparticles. J Control Release 326:192–202. https://doi.org/10.1016/j.jconrel.2020.07.004
Gandell DL, Bienen EJ, Gudeman J (2019) Mode of injection and treatment adherence: results of a survey characterizing the perspectives of health care providers and US women 18–45 years old. Patient Prefer Adherence 13:351–361. https://doi.org/10.2147/ppa.s187120
Maurizi A, Patrizii P, Teti A, Sutera FM, Baran-Rachwalska P, Burns C, Nandi U, Welsh M, Torabi-Pour N, Dehsorkhi A, Saffie-Siebert S (2023) Novel hybrid silicon–lipid nanoparticles deliver a siRNA to cure autosomal dominant osteopetrosis in mice. implications for gene therapy in humans. Mol Ther Nucleic Acids 33:925–937. https://doi.org/10.1016/j.omtn.2023.08.020
Alam I, McQueen AK, Acton D, Reilly AM, Gerard-O’Riley RL, Oakes DK, Kasipathi C, Huffer A, Wright WB, Econs MJ (2017) Phenotypic severity of autosomal dominant osteopetrosis type II (ADO2) mice on different genetic backgrounds recapitulates the features of human disease. Bone 94:34–41. https://doi.org/10.1016/j.bone.2016.10.016
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 28(1):2–17. https://doi.org/10.1002/jbmr.1805
Sherk VD, Chrisman C, Smith J, Young KC, Singh H, Bemben MG, Bemben DA (2013) Acute bone marker responses to whole-body vibration and resistance exercise in young women. J Clin Densitom 16(1):104–109. https://doi.org/10.1016/j.jocd.2012.07.009
Solberg LB, Brorson S-H, Stordalen GA, Bækkevold ES, Andersson G, Reinholt FP (2014) Increased tartrate-resistant acid phosphatase expression in osteoblasts and osteocytes in experimental osteoporosis in rats. Calcif Tissue Int 94(5):510–521. https://doi.org/10.1007/s00223-013-9834-3
Schulz A, Moshous D (2023) Hematopoietic stem cell transplantation, a curative approach in infantile osteopetrosis. Bone 167:116634. https://doi.org/10.1016/j.bone.2022.116634
Teti A (2022) Early treatment of osteopetrosis: paradigm shift to marrow cell transplantation. Bone 164:116512. https://doi.org/10.1016/j.bone.2022.116512
Ahn I, Kang CS, Han J (2023) Where should siRNAs go: applicable organs for siRNA drugs. Exp Mol Med 55(7):1283–1292. https://doi.org/10.1038/s12276-023-00998-y
The Human Protein Atlas (2023) CLCN7 (Tissue). https://www.proteinatlas.org/ENSG00000103249-CLCN7/tissue#expression_summary. Accessed 8 August 2023
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Al-Khilali Szigyarto C, Odeberg J, Djureinovic D, Ottosson Takanen J, Hober S, Alm T, Edqvist P-H, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F (2015) Tissue-based map of the human proteome. Science 347(6220):1260419. https://doi.org/10.1126/science.1260419
Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, Schmitt A, Poët M, Steinfeld R, Schweizer M, Kornak U, Jentsch TJ (2005) Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J 24(5):1079–1091. https://doi.org/10.1038/sj.emboj.7600576
Wartosch L, Fuhrmann JC, Schweizer M, Stauber T, Jentsch TJ (2009) Lysosomal degradation of endocytosed proteins depends on the chloride transport protein ClC-7. FASEB J 23(12):4056–4068. https://doi.org/10.1096/fj.09-130880
Nicoli E-R, Weston MR, Hackbarth M, Becerril A, Larson A, Zein WM, Baker PR 2nd, Burke JD, Dorward H, Davids M, Huang Y, Adams DR, Zerfas PM, Chen D, Markello TC, Toro C, Wood T, Elliott G, Vu M, Zheng W, Garrett LJ, Tifft CJ, Gahl WA, Day-Salvatore DL, Mindell JA, Malicdan MCV (2019) Lysosomal storage and albinism due to effects of a de novo CLCN7 variant on lysosomal acidification. Am J Hum Genet 104(6):1127–1138. https://doi.org/10.1016/j.ajhg.2019.04.008
Dohrn MF, Medina J, Olaciregui Dague KR, Hund E (2021) Are we creating a new phenotype? physiological barriers and ethical considerations in the treatment of hereditary transthyretin-amyloidosis. Neurol Res Pract 3(1):57. https://doi.org/10.1186/s42466-021-00155-8
Aigner A (2019) Perspectives, issues and solutions in RNAi therapy: the expected and the less expected. Nanomedicine 14(21):2777–2782. https://doi.org/10.2217/nnm-2019-0321
Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DWY, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, de Fougerolles A, Maier MA (2010) Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther 18(7):1357–1364. https://doi.org/10.1038/mt.2010.85
Seong S, Vijayan V, Kim JH, Kim K, Kim I, Cherukula K, Park I-K, Kim N (2023) Nano-formulations for bone-specific delivery of siRNA for CrkII silencing-induced regulation of bone formation and resorption to maximize therapeutic potential for bone-related diseases. Biomater Sci 11(7):2581–2589. https://doi.org/10.1039/D2BM02038F
Ladd LM, Imel EA, Niziolek PJ, Liu Z, Warden SJ, Liang Y, Econs MJ (2021) Radiographic imaging, densitometry and disease severity in autosomal dominant osteopetrosis type 2. Skeletal Radiol 50(5):903–913. https://doi.org/10.1007/s00256-020-03625-3
Garnero P (2017) The utility of biomarkers in osteoporosis management. Mol Diagn Ther 21(4):401–418. https://doi.org/10.1007/s40291-017-0272-1
Salam S, Gallagher O, Gossiel F, Paggiosi M, Khwaja A, Eastell R (2018) Diagnostic accuracy of biomarkers and imaging for bone turnover in renal osteodystrophy. J Am Soc Nephrol 29(5):1557–1565. https://doi.org/10.1681/ASN.2017050584
Desai AS, Webb DJ, Taubel J, Casey S, Cheng Y, Robbie GJ, Foster D, Huang SA, Rhyee S, Sweetser MT, Bakris GL (2023) Zilebesiran, an RNA interference therapeutic agent for hypertension. N Engl J Med 389(3):228–238
Uijl E, Mirabito Colafella KM, Sun Y, Ren L, van Veghel R, Garrelds IM, de Vries R, Poglitsch M, Zlatev I, Kim JB, Hoorn EJ, Foster D, Danser AHJ (2019) Strong and sustained antihypertensive effect of small interfering RNA targeting liver angiotensinogen. Hypertension 73(6):1249–1257
Acknowledgements
A.M. is supported by the ‘Piano Operativo Nazionale (PON) Ricerca Innovazione’ (FSE-REACT EU D.M.1062).
Funding
The work was funded by SiSaf Ltd.
Author information
Authors and Affiliations
Contributions
M.E., I.A., R.G.-O., and D.A. contributed to in vivo study design, study conduct, data analysis, and data interpretation. A.T. and A.M. designed the siRNA and contributed to assay development. S.S.-S., F.M.S., N.T.-P., A.D., P.B.-R., and L.I. contributed to study design and assay development; designed, produced, and evaluated the sshLNPs; and performed in vitro assays and organ analyses. All authors contributed to drafting and revision of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
S.S.-S. is CEO and shareholder of SiSaf Ltd. F.M.S., A.D., N.T.-P., and P.B.-R. are employees of SiSaf Ltd. A.T., A.M., L.I., and M.E. served or serve as consultants of SiSaf Ltd.
Research Involving Human and Animal Rights and Informed Consent
All procedures involving animals performed in the study followed the guidelines of the Indiana University Animal Care and Use Committee (IACUC). This study does not involve research in humans.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saffie-Siebert, S., Alam, I., Sutera, F.M. et al. Effect of Allele-Specific Clcn7G213R siRNA Delivered Via a Novel Nanocarrier on Bone Phenotypes in ADO2 Mice on 129S Background. Calcif Tissue Int (2024). https://doi.org/10.1007/s00223-024-01222-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00223-024-01222-3