Abstract
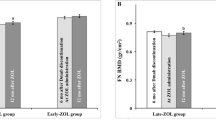
The long-term effects of zoledronate treatment in women with postmenopausal osteoporosis who stop denosumab therapy when they become osteopenic are not known. In a prospective, randomized, controlled clinical trial we previously reported that a single intravenous infusion of zoledronate 5 mg given to such patients 6 months after the last denosumab injection effectively prevents bone loss in the majority of them for up to 3 years. The study was extended for an additional 2 years and included all 19 patients from one Trial Site of the total 27 patients originally randomized in the zoledronate arm. Baseline characteristics of this cohort treated with denosumab for 2.4 ± 0.2 years were not different from those of the whole initial cohort or from the patients who did not participate in this extension. At the end of 5 years 7 patients had become again osteoporotic requiring additional treatment, 9 remained osteopenic while 3 did not complete the study extension. Thus, more than half of the osteoporotic women who became osteopenic with denosumab treatment and stopped it, maintained the BMD gains 5 years after a single zoledronate infusion with no additional treatment. Whether these results are also applicable to patients treated with denosumab for longer periods remains to be established.


Similar content being viewed by others
Data Availability
Data will be available upon reasonable request.
References
Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, Abrahamsen B, McCloskey E, Hofbauer LC, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Pepe J, Palermo A, Langdahl B (2020) Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa756
Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P (2019) Zoledronate for the prevention of bone loss in women discontinuing Denosumab treatment. A prospective 2-year clinical trial. J Bone Miner Res 34(12):2220–2228
Makras P, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Anastasilakis AD (2020) The three-year effect of a single zoledronate infusion on bone mineral density and bone turnover markers following denosumab discontinuation in women with postmenopausal osteoporosis. Bone 138:115478
Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T (2020) A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res 35(7):1207–1215
Grey A, Bolland MJ, Horne A, Wattie D, House M, Gamble G, Reid IR (2012) Five years of anti-resorptive activity after a single dose of zoledronate–results from a randomized double-blind placebo-controlled trial. Bone 50(6):1389–1393
Kendler DL, Cosman F, Stad RK, Ferrari S (2022) Denosumab in the treatment of osteoporosis: 10 years later: a narrative review. Adv Ther 39(1):58–74
Ferrari S, Libanati C, Lin CJF, Brown JP, Cosman F, Czerwinski E, de Gregomicronrio LH, Malouf-Sierra J, Reginster JY, Wang A, Wagman RB, Lewiecki EM (2019) Relationship between bone mineral density t-score and Nonvertebral fracture risk over 10 years of Denosumab treatment. J Bone Miner Res 34(6):1033–1040
Cosman F, Huang S, McDermott M, Cummings SR (2022) Multiple vertebral fractures after Denosumab discontinuation: freedom and freedom extension trials additional post hoc analyses. J Bone Miner Res 37(11):2112–2120
Sølling AS, Harsløf T, Langdahl B (2020) Treatment with Zoledronate subsequent to Denosumab in osteoporosis: a randomized trial. J Bone Miner Res 35(10):1858–1870
Makras P, Appelman-Dijkstra NM, Papapoulos SE, van Wissen S, Winter EM, Polyzos SA, Yavropoulou MP, Anastasilakis AD (2021) The duration of Denosumab treatment and the efficacy of Zoledronate to preserve bone mineral density after its discontinuation. J Clin Endocrinol Metab 106(10):e4155–e4162
Ferrari S, Langdahl B (2023) Mechanisms underlying the long-term and withdrawal effects of denosumab therapy on bone. Nat Rev Rheumatol 19(5):307–317
Burckhardt P, Faouzi M, Buclin T, Lamy O (2021) The Swiss Denosumab study, fractures after Denosumab discontinuation: a retrospective study of 797 cases. J Bone Miner Res 36(9):1717–1728
Everts-Graber J, Reichenbach S, Gahl B, Ziswiler HR, Studer U, Lehmann T (2021) Risk factors for vertebral fractures and bone loss after denosumab discontinuation: a real-world observational study. Bone 144:115830
Funding
This work was not supported by any grant, fund, or institution.
Author information
Authors and Affiliations
Contributions
Conception of the hypothesis of the study: ADA. Design of the study: ADA. Acquisition, analysis, interpretation of data: ADA, PM, SAP, SEP. Drafting the manuscript: ADA. Revising the work critically for important intellectual content: PM, SAP, SEP. Final approval of the submitted version: ADA, PM, SAP, SEP. Agreement to be accountable for all aspects of the work: ADA, PM, SAP, SEP.
Corresponding author
Ethics declarations
Conflict of interest
Athanasios D. Anastasilakis reports lecture fees from Amgen, Bianex, Eli-Lilly, Galenica, ITF, Unifarma, and UCB; Polyzois Makras reports fees for lectures/advisory boards and research grants from Amgen and fees for lectures/advisory boards from UCB, Elpen, and Galenica; Stergios A. Polyzos has nothing to declare; Socrates E. Papapoulos reports consulting/speaking fees from Amgen, Entera Bio, Qualix Dot, Radius Health, and UCB.
Ethical Approval
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the local ethics committee.
Human and Animal Rights and Informed Consent
No animals were involved in the study. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anastasilakis, A.D., Makras, P., Polyzos, S.A. et al. The Five-Year Effect of a Single Zoledronate Infusion on Bone Mineral Density Following Denosumab Discontinuation in Women with Postmenopausal Osteoporosis. Calcif Tissue Int 113, 469–473 (2023). https://doi.org/10.1007/s00223-023-01119-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01119-7