Abstract
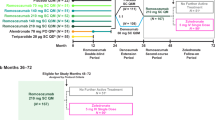
Romosozumab can increase bone mineral density (BMD) in patients with osteoporosis, but some patients do not respond to it. This study aimed to identify risk factors for being a nonresponder to romosozumab treatment. This retrospective observational study included 92 patients. Romosozumab (210 mg) was subcutaneously administered to the participants every 4 weeks over 12 months. We excluded patients who previously underwent treatment for osteoporosis to assess the impact of romosozumab alone. We evaluated the proportion of patients who did not respond to romosozumab treatment to the lumbar spine and hip with increased BMD. Nonresponders were defined as those with a bone density change of < 3% after 12 months of treatment. We compared demographics and biochemical markers between responders and nonresponders. We found that 11.5% of patients were nonresponders at the lumbar spine, and 56.8% were nonresponders at the hip. A risk factor for nonresponse at the spine was low type I procollagen N-terminal propeptide (P1NP) values at 1 month. The cutoff value for P1NP at month 1 was 50 ng/ml. We found that 11.5% and 56.8% of patients experienced no significant improvement in the lumbar spine and hip BMD, respectively. Clinicians should use nonresponse risk factors to inform decisions about romosozumab treatment for patients with osteoporosis.
Similar content being viewed by others
References
Lems WF, Raterman HG (2017) Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Ther Adv Musculoskelet Dis 9:299–316. https://doi.org/10.1177/1759720X17732562
Anam AK, Insogna K, Anam A, Insogna K (2021) Update on osteoporosis screening and management. Medical Clin N 105:1117–1134. https://doi.org/10.1016/j.mcna.2021.05.016
Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jönsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42. https://doi.org/10.1007/s00198-003-1490-4
Morin S, Lix LM, Azimaee M, Metge C, Caetano P, Leslie WD (2011) Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos Int 22:2439–2448. https://doi.org/10.1007/s00198-010-1480-2
Soen S, Usuba K, Crawford B, Adachi K (2021) Family caregiver burden of patients with osteoporotic fracture in Japan. J Bone Miner Metab 39:612–622. https://doi.org/10.1007/s00774-020-01197-9
Sambrook PN, Cameron ID, Chen JS, March LM, Simpson JM, Cumming RG, Seibel MJ (2011) Oral bisphosphonates are associated with reduced mortality in frail older people: a prospective five-year study. Osteoporos Int 22:2551–2556. https://doi.org/10.1007/s00198-010-1444-6
Iida H, Sakai Y, Seki T, Watanabe T, Wakao N, Matsui H, Imagama S (2022) Bisphosphonate treatment is associated with decreased mortality rates in patients after osteoporotic vertebral fracture. Osteoporos Int. https://doi.org/10.1007/s00198-021-06264-z
Balemans W (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543. https://doi.org/10.1093/hmg/10.5.537
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CAF, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543. https://doi.org/10.1056/NEJMoa1607948
Tominaga A, Wada K, Okazaki K, Nishi H, Terayama Y, Kato Y (2021) Early clinical effects, safety, and predictors of the effects of romosozumab treatment in osteoporosis patients: one-year study. Osteoporos Int 32:1999–2009. https://doi.org/10.1007/s00198-021-05925-3
Ebina K, Tsuboi H, Nagayama Y, Kashii M, Kaneshiro S, Miyama A, Nakaya H, Kunugiza Y, Hirao M, Okamura G, Etani Y, Takami K, Goshima A, Miura T, Nakata K, Okada S (2021) Effects of prior osteoporosis treatment on 12-month treatment response of romosozumab in patients with postmenopausal osteoporosis. Joint Bone Spine 88:105219. https://doi.org/10.1016/j.jbspin.2021.105219
Kobayakawa T, Suzuki T, Nakano M, Saito M, Miyazaki A, Takahashi J, Nakamura Y (2021) Real-world effects and adverse events of romosozumab in Japanese osteoporotic patients: a prospective cohort study. Bone Rep 14:101068. https://doi.org/10.1016/j.bonr.2021.101068
Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, Dokoupilova E, Engelke K, Finkelstein JS, Genant HK, Goemaere S, Hyldstrup L, Jodar-Gimeno E, Keaveny TM, Kendler D, Lakatos P, Maddox J, Malouf J, Massari FE, Molina JF, Ulla MR, Grauer A (2017) Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 390:1585–1594. https://doi.org/10.1016/S0140-6736(17)31613-6
Cosman F, Kendler DL, Langdahl BL, Leder BZ, Lewiecki EM, Miyauchi A, Rojeski M, McDermott M, Oates MK, Milmont CE, Libanati C, Ferrari S (2022) Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int 33:1243–1256. https://doi.org/10.1007/s00198-021-06174-0
Roman SA, Roman S, Sosa J, Pietrzak R, Snyder P, Thomas D, Udelsman R, Mayes L et al (2011) The effects of serum calcium and parathyroid hormone changes on psychological and cognitive function in patients undergoing parathyroidectomy for primary hyperparathyroidism. Ann Surg 253:131–137. https://doi.org/10.1097/SLA.0b013e3181f66720
Holick MF (2017) The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 18:153–165. https://doi.org/10.1007/s11154-017-9424-1
Tothill P, Fenner JAK, Reid DM, Tothill P, Fenner JAK, Reid DM (1995) Comparisons between three dual-energy X-ray absorptiometers used for measuring spine and femur. Br J Radiol 68:621–629. https://doi.org/10.1259/0007-1285-68-810-621
Cavalier E, Souberbielle JC, Gadisseur R, Dubois B, Krzesinski JM, Delanaye P (2014) Inter-method variability in bone alkaline phosphatase measurement: clinical impact on the management of dialysis patients. Clin Biochem 47:1227–1230. https://doi.org/10.1016/j.clinbiochem.2014.04.007
Eastell R, Krege JH, Chen P, Glass EV, Reginster JY (2006) Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr Med Res Opin 22:61–66. https://doi.org/10.1185/030079905X75096
Igarashi Y, Lee MY, Matsuzaki S (2002) Acid phosphatases as markers of bone metabolism. J Chromatogr B Analyt Technol Biomed Life Sci 781:345–358. https://doi.org/10.1016/s1570-0232(02)00431-2
Gertz BJ, Clemens JD, Holland SD, Yuan W, Greenspan S (1998) Application of a new serum assay for type I collagen cross-linked n-telopeptides: assessment of diurnal changes in bone turnover with and without alendronate treatment. Calcif Tissue Int 63:102–106. https://doi.org/10.1007/s002239900497
Garnero P, Vergnaud P, Hoyle N, Garnero P, Vergnaud P, Hoyle N (2008) Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem 54:188–196. https://doi.org/10.1373/clinchem.2007.094953
Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET (2017) Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int 28:2541–2556. https://doi.org/10.1007/s00198-017-4082-4
Yamada S, Inaba M, Kurajoh M, Shidara K, Imanishi Y, Ishimura E, Nishizawa Y (2008) Utility of serum tartrate-resistant acid phosphatase (TRACP5b) as a bone resorption marker in patients with chronic kidney disease: independence from renal dysfunction. Clin Endocrinol (Oxf) 69:189–196. https://doi.org/10.1111/j.1365-2265.2008.03187.x
Fogelman I, Blake GM (2000) Different approaches to bone densitometry. J Nucl Med 41:2015–2025
Gallagher JC, Rosen CJ, Chen P, Misurski DA, Marcus R (2006) Response rate of bone mineral density to teriparatide in postmenopausal women with osteoporosis. Bone 39:1268–1275. https://doi.org/10.1016/j.bone.2006.06.007
Miller PD, Hattersley G, Lau E, Fitzpatrick LA, Harris AG, Williams GC, Hu MY, Riis BJ, Russo L, Christiansen C (2019) Bone mineral density response rates are greater in patients treated with abaloparatide compared with those treated with placebo or teriparatide: Results from the ACTIVE phase 3 trial. Bone 120:137–140. https://doi.org/10.1016/j.bone.2018.10.015
Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A (2016) A retrospective analysis of nonresponse to daily teriparatide treatment. Osteoporos Int 27:2845–2853. https://doi.org/10.1007/s00198-016-3581-z
Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K, Matsumoto T, Milmont CE, Libanati C, Grauer A (2018) FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res 33:1219–1226. https://doi.org/10.1002/jbmr.3427
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Ishibashi H, Ishibashi H, Crittenden D, Miyauchi A, Libanati C, Maddox J, Fan M, Chen L, Grauer A et al (2017) Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone (New York, NY) 103:209–215. https://doi.org/10.1016/j.bone.2017.07.005
Takada J, Dinavahi R, Miyauchi A, Hamaya E, Hirama T, Libanati C, Nakamura Y, Milmont CE, Grauer A (2020) Relationship between P1NP, a biochemical marker of bone turnover, and bone mineral density in patients transitioned from alendronate to romosozumab or teriparatide: a post hoc analysis of the STRUCTURE trial. J Bone Miner Metab 38:310–315. https://doi.org/10.1007/s00774-019-01057-1
Ominsky MS, Niu Q-T, Li C, Li X, Ke HZ (2014) Tissue-level mechanisms responsible for the increase in bone formation and bone volume by sclerostin antibody. J Bone Miner Res 29:1424–1430. https://doi.org/10.1002/jbmr.2152
Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK (2011) Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int 22:357–362. https://doi.org/10.1007/s00198-010-1226-1
Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH (2011) PINP as an aid for monitoring patients treated with teriparatide. Bone 48:798–803. https://doi.org/10.1016/j.bone.2010.12.006
Shimizu T, Arita K, Murota E, Hiratsuka S, Fujita R, Ishizu H, Asano T, Takahashi D, Takahata M, Iwasaki N (2021) Effects after starting or switching from bisphosphonate to romosozumab or denosumab in Japanese postmenopausal patients. J Bone Miner Metab 39:868–875. https://doi.org/10.1007/s00774-021-01226-1
Tominaga A, Wada K, Kato Y, Nishi H, Terayama Y, Okazaki K (2021) Early clinical effects, safety, and appropriate selection of bone markers in romosozumab treatment for osteoporosis patients: a 6-month study. Osteoporos Int 32:653–661. https://doi.org/10.1007/s00198-020-05639-y
Acknowledgements
The authors would like to thank Enago (www.enago.com) for the manuscript review and editing support.
Funding
This work was partly supported by a research grant from Tokyo Women’s Medical University Career Development Center for Medical Professionals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ayako Tominaga, Ken Okazaki, Hideharu Nishi, Yasushi Terayama, Shuji Shimamoto, Yasuteru Kodama, and Yoshiharu Kato declare that they have no conflicts of interest. Keiji Wada received a speaking fee from Amgen Inc.
Ethical approval
The procedures complied with the 1964 Helsinki declaration and its later amendments and were approved by Tokyo Women’s Medical University Ethics Committee, number 5596.
Informed consent
Informed consent was obtained from all participants in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tominaga, A., Wada, K., Okazaki, K. et al. Nonresponder Considerations for Romosozumab Treatment. Calcif Tissue Int 113, 157–165 (2023). https://doi.org/10.1007/s00223-023-01087-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01087-y