Abstract
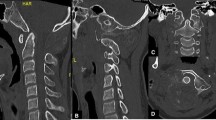
Aneurysmal bone cysts (ABC) are rare osteolytic, benign but often locally aggressive tumours of the long bones or vertebrae. For spinal ABC, surgical management, embolisation or sclerotherapy alone often carry high morbidity and/or high recurrence rates. Interruption of receptor activator of nuclear factor-kappa B ligand (RANKL) signalling holds promise as an effective therapeutic strategy for these tumours. We aimed to review the approach to surgical management and evaluate the efficacy and safety of denosumab for ABC of the spine in children. Retrospective review of 7 patients treated with denosumab using a standardised protocol for ABC of the spine in a tertiary paediatric centre. Surgical intervention was only conducted if there was spinal instability or significant neurological impairment. Denosumab 70 mg/m2 was given 4-weekly for at least 6 months, followed by 2 doses of zoledronate 0.025 mg/kg, aiming to prevent rebound hypercalcaemia. All patients achieved stability of the spine and resolution of neurological impairment, if present. Six patients achieved metabolic remission and have ceased denosumab without recurrence to date; the other showed clinical and radiological improvement without complete metabolic remission. Three patients developed symptomatic hypercalcaemia 5–7 months after cessation of denosumab, requiring additional bisphosphonate treatment. We present our algorithm for the surgical and medical management of paediatric spinal ABC. Denosumab produced a radiological and metabolic response in all patients, with complete remission in most. Follow-up time was not long enough to evaluate the endurance of response after cessation in some patients. Incidence of rebound hypercalcaemia in this paediatric cohort was high, prompting a change to our protocol.



Similar content being viewed by others
References
Rapp TB, Ward JP, Alaia MJ (2012) Aneurysmal bone cyst. J Am Acad Orthop Surg 20:233–241. https://doi.org/10.5435/JAAOS-20-04-233
Lange T, Stehling C, Frohlich B, Klingenhofer M, Kunkel P, Schneppenheim R, Escherich G, Gosheger G, Hardes J, Jurgens H, Schulte TL (2013) Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J 22:1417–1422. https://doi.org/10.1007/s00586-013-2715-7
Durr HR, Grahneis F, Baur-Melnyk A, Knosel T, Birkenmaier C, Jansson V, Klein A (2019) Aneurysmal bone cyst: results of an off label treatment with Denosumab. BMC Musculoskelet Disord 20:456. https://doi.org/10.1186/s12891-019-2855-y
Kurucu N, Akyuz C, Ergen FB, Yalcin B, Kosemehmetoglu K, Ayvaz M, Varan A, Aydin B, Kutluk T (2018) Denosumab treatment in aneurysmal bone cyst: evaluation of nine cases. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.26926
Sciot R, Dorfman H, Brys P, Dal Cin P, De Wever I, Fletcher CD, Jonson K, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Samson I, Tallini G, Van den Berghe H, Vanni R, Willen H (2000) Cytogenetic-morphologic correlations in aneurysmal bone cyst, giant cell tumor of bone and combined lesions. a report from the CHAMP study group. Mod Pathol 13:1206–1210. https://doi.org/10.1038/modpathol.3880224
Palmerini E, Ruggieri P, Angelini A, Boriani S, Campanacci D, Milano GM, Cesari M, Paioli A, Longhi A, Abate ME, Scoccianti G, Terzi S, Trovarelli G, Franchi A, Picci P, Ferrari S, Leopardi MP, Pierini M (2018) Denosumab in patients with aneurysmal bone cysts: a case series with preliminary results. Tumori 104:344–351. https://doi.org/10.1177/0300891618784808
Oliveira AM, Chou MM (2014) USP6-induced neoplasms: the biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum Pathol 45:1–11. https://doi.org/10.1016/j.humpath.2013.03.005
Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD, Federman N, Bernthal NM (2016) Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med 9:435–444. https://doi.org/10.1007/s12178-016-9371-6
Maximen J, Robin F, Tronchot A, Rossetti A, Ropars M, Guggenbuhl P (2022) Denosumab in the management of Aneurysmal bone cyst: a literature review. Joint Bone Spine 89:105260. https://doi.org/10.1016/j.jbspin.2021.105260
Pelle DW, Ringler JW, Peacock JD, Kampfschulte K, Scholten DJ 2nd, Davis MM, Mitchell DS, Steensma MR (2014) Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res 164:139–148. https://doi.org/10.1016/j.trsl.2014.03.005
Won KY, Kalil RK, Kim YW, Park YK (2011) RANK signalling in bone lesions with osteoclast-like giant cells. Pathology 43:318–321. https://doi.org/10.1097/PAT.0b013e3283463536
Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay J-Y, Roudier M, Smith J, Ye Z, Sohn W, Dansey R, Jun S (2010) Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 11:275–280. https://doi.org/10.1016/s1470-2045(10)70010-3
Engellau J, Seeger L, Grimer R, Henshaw R, Gelderblom H, Choy E, Chawla S, Reichardt P, O’Neal M, Feng A, Jacobs I, Roberts ZJ, Braun A, Bach BA (2018) Assessment of denosumab treatment effects and imaging response in patients with giant cell tumor of bone. World J Surg Oncol 16:191. https://doi.org/10.1186/s12957-018-1478-3
Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, Grimer RJ, Choy E, Skubitz K, Seeger L, Schuetze SM, Henshaw R, Dai T, Jandial D, Palmerini E (2019) Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol 20:1719–1729. https://doi.org/10.1016/S1470-2045(19)30663-1
Boyce AM (2017) Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep 15:283–292. https://doi.org/10.1007/s11914-017-0380-1
Choe M, Smith V, Okcu MF, Wulff J, Gruner S, Huisman T, Venkatramani R (2021) Treatment of central giant cell granuloma in children with denosumab. Pediatr Blood Cancer 68:e28778. https://doi.org/10.1002/pbc.28778
Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH, Harrop JS, Fehlings MG, Boriani S, Chou D, Schmidt MH, Polly DW, Biagini R, Burch S, Dekutoski MB, Ganju A, Gerszten PC, Gokaslan ZL, Groff MW, Liebsch NJ, Mendel E, Okuno SH, Patel S, Rhines LD, Rose PS, Sciubba DM, Sundaresan N, Tomita K, Varga PP, Vialle LR, Vrionis FD, Yamada Y, Fourney DR (2010) A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine oncology study group. Spine (Phila Pa 1976) 35:E1221-1229. https://doi.org/10.1097/BRS.0b013e3181e16ae2
Vanderniet JA, Wall CL, Mullins A, London K, Lim L, Hibbert S, Briody J, Padhye B, Poon M, Biggin A, Dalla-Pozza L, Munns CF (2022) Denosumab for central giant cell granuloma in an Australian tertiary paediatric centre. Bone 159:116395. https://doi.org/10.1016/j.bone.2022.116395
Ooi HL, Briody J, Biggin A, Cowell CT, Munns CF (2013) Intravenous zoledronic acid given every 6 months in childhood osteoporosis. Horm Res Paediatr 80:179–184. https://doi.org/10.1159/000354303
Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR (2004) Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 4:451–464. https://doi.org/10.1016/j.spinee.2003.07.007
Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte W, Harrop JS, Aarabi B, Shaffrey C, Dvorak M, Fisher C, Arnold P, Massicotte EM, Lewis S, Rampersaud R (2012) Early versus delayed decompression for traumatic cervical spinal cord injury: results of the surgical timing in acute spinal cord injury study (STASCIS). PLoS ONE 7:e32037. https://doi.org/10.1371/journal.pone.0032037
Wilson JR, Singh A, Craven C, Verrier MC, Drew B, Ahn H, Ford M, Fehlings MG (2012) Early versus late surgery for traumatic spinal cord injury: the results of a prospective Canadian cohort study. Spinal Cord 50:840–843. https://doi.org/10.1038/sc.2012.59
Papagelopoulos PJ, Choudhury SN, Frassica FJ, Bond JR, Unni KK, Sim FH (2001) Treatment of aneurysmal bone cysts of the pelvis and sacrum. J Bone Joint Surg Am 83:1674–1681. https://doi.org/10.2106/00004623-200111000-00009
Alhumaid I, Abu-Zaid A (2019) Denosumab therapy in the management of aneurysmal bone cysts: a comprehensive literature review. Cureus 11:e3989. https://doi.org/10.7759/cureus.3989
Boye K, Jebsen NL, Zaikova O, Knobel H, Londalen AM, Trovik CS, Monge OR, Hall KS (2017) Denosumab in patients with giant-cell tumor of bone in Norway: results from a nationwide cohort. Acta Oncol 56:479–483. https://doi.org/10.1080/0284186X.2016.1278305
Muheremu A, Ma Y, Huang Z, Shan H, Li Y, Niu X (2017) Diagnosing giant cell tumor of the bone using positron emission tomography/computed tomography: a retrospective study of 20 patients from a single center. Oncol Lett 14:1985–1988. https://doi.org/10.3892/ol.2017.6379
Hao K, Wang Q (2019) 18F-FDG PET/CT imaging in evaluation the efficacy of denosumab for giant cell tumor of bone. J Nucl Med 60:1279–1279
Pan KS, Boyce AM (2021) Denosumab treatment for giant cell tumors, aneurysmal bone cysts, and fibrous dysplasia-risks and benefits. Curr Osteoporos Rep 19:141–150. https://doi.org/10.1007/s11914-021-00657-z
Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, Bassim C, Cherman N, Ellsworth M, Kasa-Vubu JZ, Farley FA, Molinolo AA, Bhattacharyya N, Collins MT (2012) Denosumab treatment for fibrous dysplasia. J Bone Miner Res 27:1462–1470. https://doi.org/10.1002/jbmr.1603
Grasemann C, Schundeln MM, Hovel M, Schweiger B, Bergmann C, Herrmann R, Wieczorek D, Zabel B, Wieland R, Hauffa BP (2013) Effects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget’s disease. J Clin Endocrinol Metab 98:3121–3126. https://doi.org/10.1210/jc.2013-1143
Gossai N, Hilgers MV, Polgreen LE, Greengard EG (2015) Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Cancer 62:1078–1080. https://doi.org/10.1002/pbc.25393
Setsu N, Kobayashi E, Asano N, Yasui N, Kawamoto H, Kawai A, Horiuchi K (2016) Severe hypercalcemia following denosumab treatment in a juvenile patient. J Bone Miner Metab 34:118–122. https://doi.org/10.1007/s00774-015-0677-z
Uday S, Gaston CL, Rogers L, Parry M, Joffe J, Pearson J, Sutton D, Grimer R, Hogler W (2018) Osteonecrosis of the jaw and rebound hypercalcemia in young people treated with denosumab for giant cell tumor of bone. J Clin Endocrinol Metab 103:596–603. https://doi.org/10.1210/jc.2017-02025
Sydlik C, Durr HR, Pozza SB, Weissenbacher C, Roeb J, Schmidt H (2020) Hypercalcaemia after treatment with denosumab in children: bisphosphonates as an option for therapy and prevention? World J Pediatr 16:520–527. https://doi.org/10.1007/s12519-020-00378-w
Del Sindaco G, Berlanga P, Brugieres L, Thebault E, Mantovani G, Wicart P, Linglart A (2021) Mineral and bone consequences of high dose denosumab therapy to treat an aneurysmal bone cyst, a child case report. Front Endocrinol (Lausanne) 12:698963. https://doi.org/10.3389/fendo.2021.698963
Horiuchi K, Kobayashi E, Mizuno T, Susa M, Chiba K (2021) Hypercalcemia following discontinuation of denosumab therapy: a systematic review. Bone Rep 15:101148. https://doi.org/10.1016/j.bonr.2021.101148
Wang HD, Boyce AM, Tsai JY, Gafni RI, Farley FA, Kasa-Vubu JZ, Molinolo AA, Collins MT (2014) Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab 99:891–897. https://doi.org/10.1210/jc.2013-3081
Kieser DC, Mazas S, Cawley DT, Fujishiro T, Tavolaro C, Boissiere L, Obeid I, Pointillart V, Vital JM, Gille O (2018) Bisphosphonate therapy for spinal aneurysmal bone cysts. Eur Spine J 27:851–858. https://doi.org/10.1007/s00586-018-5470-y
Cornelis F, Truchetet ME, Amoretti N, Verdier D, Fournier C, Pillet O, Gille O, Hauger O (2014) Bisphosphonate therapy for unresectable symptomatic benign bone tumors: a long-term prospective study of tolerance and efficacy. Bone 58:11–16. https://doi.org/10.1016/j.bone.2013.10.004
Simm PJ, O’Sullivan M, Zacharin MR (2013) Successful treatment of a sacral aneurysmal bone cyst with zoledronic acid. J Pediatr Orthop 33:e61-64. https://doi.org/10.1097/BPO.0b013e318285c3a7
Acknowledgements
Other members of Denosumab Treatment Group: Brian Hsu, Nathan Hartin, Vinay Kulkarni, David Lord, Richard Widmer.
Funding
No external funding was received for this project.
Author information
Authors and Affiliations
Contributions
JAV: conceptualisation, methodology, data collection, analysis, writing—original draft; DT: conceptualisation, data collection, analysis, writing—original draft; CLW: conceptualisation, methodology, data collection, writing—review & editing; CMG: data collection, writing—review & editing; KL: writing—review & editing; CK—writing—review & editing; SH: writing—review & editing; JB: data curation, writing—review & editing; MP: writing—review & editing; BP: writing—review & editing; AB: visualisation, writing—review & editing; LDP: writing—review & editing; RJG: conceptualisation, writing—review & editing, supervision; CFM: conceptualisation, methodology, writing—review & editing, supervision.
Corresponding author
Ethics declarations
Denosumab was administered following approval from the Sydney Children's Hospitals Network Drug Committee.
Conflict of interest
Joel A. Vanderniet, Dionysios Tsinas, Christie-Lee Wall, Christian M. Girgis, Kevin London, Corinne Keane, Julie Briody, Sally Hibbert, Myra Poon, Bhavna Padhye, Andrew Biggin, Luciano Dalla-Pozza, Randolph J. Gray and Craig F. Munns have no conflicts of interest to declare.
Ethical approval
This study was approved by the Human Research Ethics Committee at our institution (2019/ETH13024) and conducted in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was not required for this retrospective review of clinical data, in which no identifying details were published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vanderniet, J.A., Tsinas, D., Wall, CL. et al. Surgical Management and Denosumab for Aneurysmal Bone Cysts of the Spine in an Australian Tertiary Paediatric Centre. Calcif Tissue Int 112, 592–602 (2023). https://doi.org/10.1007/s00223-023-01068-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01068-1