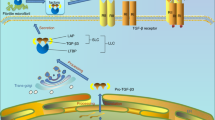
Abstract
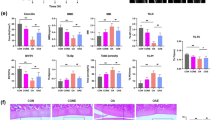
Osteoarthritis (OA) is a major health problem, characterized by progressive cartilage degeneration. Previous works have shown that mechanical loading can alleviate OA symptoms by suppressing catabolic activities. This study evaluated whether mechanical loading can enhance anabolic activities by facilitating the recruitment of stem cells for chondrogenesis. We evaluated cartilage degradation in a mouse model of OA through histology with H&E and safranin O staining. We also evaluated the migration and chondrogenic ability of stem cells using in vitro assays, including immunohistochemistry, immunofluorescence, and Western blot analysis. The result showed that the OA mice that received mechanical loading exhibited resilience to cartilage damage. Compared to the OA group, mechanical loading promoted the expression of Piezo1 and the migration of stem cells was promoted via the SDF-1/CXCR4 axis. Also, the chondrogenic differentiation was enhanced by the upregulation of SOX9, a transcription factor important for chondrogenesis. Collectively, the results revealed that mechanical loading facilitated cartilage repair by promoting the migration and chondrogenic differentiation of endogenous stem cells. This study provided new insights into the loading-driven engagement of endogenous stem cells and the enhancement of anabolic responses for the treatment of OA.








Similar content being viewed by others
References
LaTourette A, Kloppenburg M, Richette P (2020) Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol 16(12):673–688
Zhang Y, Chen X, Tong Y et al (2020) Development and prospect of intra-articular injection in the treatment of osteoarthritis: a review. J Pain Res 13:1941–1955
Wan Y, Li W, Liao Z et al (2020) Selective MMP-13 inhibitors: promising agents for the therapy of osteoarthritis. Curr Med Chem 27(22):3753–3769
Billesberger LM, Fisher KM, Qadri YJ et al (2020) Procedural treatments for knee osteoarthritis: a review of current injectable therapies. Pain Res Manag 2020:3873098
Maheshwer B, Polce EM, Paul K et al (2021) Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: a systematic review and meta-analysis. Arthroscopy 37(1):362–378
Yang Z, Li H, Yuan Z et al (2020) Endogenous cell recruitment strategy for articular cartilage regeneration. Acta Biomater 114:31–52
Luo Z, Jiang L, Xu Y et al (2015) Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials 52:463–475
McGonagle D, Baboolal TG, Jones E (2017) Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol 13(12):719–730
Salinas EY, Aryaei A, Paschos N et al (2020) Shear stress induced by fluid flow produces improvements in tissue-engineered cartilage. Biofabrication 12(4):045010
Zhao Z, Li Y, Wang M et al (2020) Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J Cell Mol Med 24(10):5408–5419
Chen L, Huang T, Qiao Y et al (2019) Perspective into the regulation of cell-generated forces toward stem cell migration and differentiation. J Cell Biochem 120(6):8884–8890
Ye W, Guo H, Yang X et al (2020) Pulsed electromagnetic field versus whole body vibration on cartilage and subchondral trabecular bone in mice with knee osteoarthritis. Bioelectromagnetics 41(4):298–307
Huwe LW, Sullan GK, Hu JC et al (2017) Using costal chondrocytes to engineer articular cartilage with applications of passive axial compression and bioactive stimuli. Tissue Eng Part A 24(5–6):516–526
Antunes BP, Vainieri ML, Alini M et al (2020) Enhanced chondrogenic phenotype of primary bovine articular chondrocytes in Fibrin-Hyaluronan hydrogel by multi-axial mechanical loading and FGF18. Acta Biomater 105:170–179
Zhang P, Yokota H (2012) Elbow loading promotes longitudinal bone growth of the ulna and the humerus. J Bone Miner Metab 30(1):31–39
Zheng W, Ding B, Li X et al (2020) Knee loading repairs osteoporotic osteoarthritis by relieving abnormal remodeling of subchondral bone via Wnt/β-catenin signaling. FASEB J 34(2):3399–3412
Yang S, Liu H, Zhu L et al (2019) Ankle loading ameliorates bone loss from breast cancer-associated bone metastasis. FASEB J 33(10):10742–10752
Zhang P, Hamamura K, Yokota H et al (2009) Potential applications of pulsating joint loading in sports medicine. Exerc Sport Sci Rev 37(1):52–56
Zhang P, Yokota H (2011) Knee loading stimulates healing of mouse bone wounds in a femur neck. Bone 49(4):867–872
Zhang P, Sun Q, Turner CH et al (2007) Knee loading accelerates bone healing in mice. J Bone Miner Res 22(12):1979–1987
Liu D, Li X, Li J et al (2015) Knee loading protects against osteonecrosis of the femoral head by enhancing vessel remodeling and bone healing. Bone 81:620–631
Zheng W, Li X, Liu D et al (2019) Mechanical loading mitigates osteoarthritis symptoms by regulating endoplasmic reticulum stress and autophagy. FASEB J 33(3):4077–4088
Li X, Yang J, Liu D et al (2016) Knee loading inhibits osteoclast lineage in a mouse model of osteoarthritis. Sci Rep 6:24668
Wang HN, Huang YC, Ni GX (2020) Mechanotransduction of stem cells for tendon repair. World J Stem Cells 12(9):952–965
Martino F, Perestrelo AR, Vinarský V et al (2018) Cellular mechanotransduction: from tension to function. Front Physiol 9:824
Xu X, Liu S, Liu H et al (2021) Piezo channels: awesome mechanosensitive structures in cellular mechanotransduction and their role in bone. Int J Mol Sci 22(12):6429
Lewis AH, Grandl J (2020) Inactivation kinetics and mechanical gating of Piezo1 ion channels depend on subdomains within the cap. Cell Rep 30(3):870–880
Zhang L, Liu X, Gao L et al (2020) Activation of Piezo1 by ultrasonic stimulation and its effect on the permeability of human umbilical vein endothelial cells. Biomed Pharmacother 131:110796
Sun Y, Leng P, Li D et al (2020) Mechanism of abnormal chondrocyte proliferation induced by Piezo1-siRNA exposed to mechanical stretch. Biomed Res Int 2020:8538463
Nam D, Park A, Dubon MJ et al (2020) Coordinated regulation of mesenchymal stem cell migration by various chemotactic stimuli. Int J Mol Sci 21(22):8561
Tatara AM, Kretlow JD, Spicer PP et al (2015) Autologously generated tissue-engineered bone flaps for reconstruction of large mandibular defects in an ovine model. Tissue Eng Part A 21(9–10):1520–1528
Li X, Wei Z, Lv H et al (2019) Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int J Nanomedicine 14:573–589
Barhanpurkar-Naik A, Mhaske ST, Pote ST et al (2017) Interleukin-3 enhances the migration of human mesenchymal stem cells by regulating expression of CXCR4. Stem Cell Res Ther 8(1):168
Wang Y, Sun X, Lv J et al (2017) Stromal cell-derived factor-1 accelerates cartilage defect repairing by recruiting bone marrow mesenchymal stem cells and promoting chondrogenic differentiation. Tissue Eng Part A 23(19–20):1160–1168
Kuang B, Zeng Z, Qin Q (2019) Biomechanically stimulated chondrocytes promote osteoclastic bone resorption in the mandibular condyle. Arch Oral Biol 98:248–257
Zhang Y, Li X, Li J et al (2022) Knee loading enhances the migration of adipose-derived stem cells to the osteoarthritic sites through the SDF-1/CXCR4 regulatory axis. Calcif Tissue Int 111(2):171–184
Almalki SG, Agrawal DK (2016) Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 92(1–2):41–51
Horner CB, Hirota K, Liu J et al (2018) Magnitude-dependent and inversely-related osteogenic/chondrogenic differentiation of human mesenchymal stem cells under dynamic compressive strain. J Tissue Eng Regen Med 12(2):e637–e647
Berenbaum F, Walker C (2020) Osteoarthritis and inflammation: a serious disease with overlapping phenotypic patterns. Postgrad Med 132(4):377–384
Costello CA, Hu T, Liu M et al (2020) Metabolomics signature for non-responders to total joint replacement surgery in primary osteoarthritis patients: the newfoundland osteoarthritis study. J Orthop Res 38(4):793–802
Davatchi F, Sadeghi AB, Mohyeddin M et al (2016) Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis 19(3):219–225
Lu J, Shen X, Sun X et al (2018) Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics 8(18):5039–5058
Im GI (2016) Endogenous cartilage repair by recruitment of stem cells. Tissue Eng Part B Rev 22(2):160–171
Bourzac C, Bensidhoum M, Pallu S et al (2019) Use of adult mesenchymal stromal cells in tissue repair: impact of physical exercise. Am J Physiol Cell Physiol 317(4):C642–C654
Xiang X, Liu H, Wang L et al (2020) Ultrasound combined with SDF-1α chemotactic microbubbles promotes stem cell homing in an osteoarthritis model. J Cell Mol Med 24(18):10816–10829
Zhao Z, Wang Y, Wang Q et al (2021) Radial extracorporeal shockwave promotes subchondral bone stem/progenitor cell self-renewal by activating YAP/TAZ and facilitates cartilage repair in vivo. Stem Cell Res Ther 12(1):19
Tian GE, Zhou JT, Liu XJ et al (2019) Mechanoresponse of stem cells for vascular repair. World J Stem Cells 11(12):1104–1114
Lee W, Leddy HA, Chen Y et al (2014) Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A 111(47):E5114-5122
Mousawi F, Peng H, Li J et al (2020) Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells 38(3):410–421
Sugimoto A, Miyazaki A, Kawarabayashi K et al (2017) Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep 7(1):17696
Shang F, Yu Y, Liu S et al (2020) Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact Mater 6(3):666–683
Yuan L, Sakamoto N, Song G et al (2012) Low-level shear stress induces human mesenchymal stem cell migration through the SDF-1/CXCR4 axis via MAPK signaling pathways. Stem Cells Dev 22(17):2384–2393
Zhang X, Wu S, Zhu Y et al (2021) Exploiting joint-resident stem cells by exogenous SOX9 for cartilage regeneration for therapy of osteoarthritis. Front Med 8:622609
Jang Y, Jung H, Nam Y et al (2016) Centrifugal gravity-induced BMP4 induces chondrogenic differentiation of adipose-derived stem cells via SOX9 upregulation. Stem Cell Res Ther 7(1):184
Carroll SF, Buckley CT, Kelly DJ (2014) Cyclic hydrostatic pressure promotes a stable cartilage phenotype and enhances the functional development of cartilaginous grafts engineered using multipotent stromal cells isolated from bone marrow and infrapatellar fat pad. J Biomech 47(9):2115–2121
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81772405 and 81572100 to P. Zhang; 81601863 to X. Li).
Funding
National Natural Science Foundation of China,81772405,Ping Zhang,81572100,Ping Zhang,81601863,Xinle Li
Author information
Authors and Affiliations
Contributions
PZ, JL, and XW designed research; XW, JL, RK and XL conducted research; DL, LZ, LY, RK and XW collected the data; JL, XW, XL, RK, LY, HY, and PZ analyzed the data; PZ JL, and XW wrote the manuscript; PZ approved the final manuscript as submitted; PZ accepted responsibility for the integrity of data analysis.
Corresponding author
Ethics declarations
Competing interest
Jie Li, Xiaoyu Wang, Xinle Li, Daquan Liu, Lidong Zhai, Xuetong Wang, Ran Kang, Hiroki Yokota, Lei Yang, Ping Zhang declare that they have no competing interests.
Resaerch Involving Human and Animal Rights
All experiments were carried out according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Ethics Committee of Tianjin Medical University. No human studies were performed in the course of these experiments.
Informed Consent
For this type of study, no informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Wang, X., Li, X. et al. Mechanical Loading Promotes the Migration of Endogenous Stem Cells and Chondrogenic Differentiation in a Mouse Model of Osteoarthritis. Calcif Tissue Int 112, 363–376 (2023). https://doi.org/10.1007/s00223-022-01052-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01052-1