Abstract
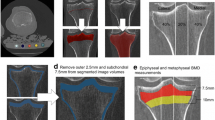
Subchondral bone properties are associated with the pathogenesis of osteoarthritis (OA), but this relationship has not been confirmed in the trapeziometacarpal joint (TMCJ). We aimed to evaluate the thickness (SBT) and density (SBD) of three-dimensional (3D) trapezium subchondral bone models derived from computed tomography (CT) images, and their relationships with early-stage TMCJ OA. We reviewed patients with a distal radius fracture who underwent conventional CT scans and such osteoporosis evaluations as bone mineral density (BMD) and bone turnover markers (BTMs). From 3D trapezium subchondral bone models, we measured SBT and SBD according to the OA stage and performed multivariate analyses to evaluate their associations with age, sex, body mass index, BMD, and BTMs. As results, a total of 156 patients (78 men and 78 age-matched women; mean age, 67 ± 10 years) were analyzed. There were 30 (19%) with grade 0, 71 (45%) with grade 1, 13 (8%) with grade 2, and 42 (27%) with grade 3 TMCJ OA. SBT was significantly lower in patients with grade 1 OA than those with grade 0 or grade 3 OA, but SBD generally increased according to the OA severity. Low SBT was associated with low BMD, and low SBD with low BMD, high osteocalcin levels, and severe OA grades. In conclusion, patients with early-stage radiographic TMCJ OA have a lower SBT at the trapezium, which may support the potential role of subchondral bone in OA pathogenesis. This study also shows that subchondral bone properties are associated with BMD and osteocalcin levels.




Similar content being viewed by others
References
Abramson SB, Attur M, Yazici Y (2006) Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheumatol 2:304–312
Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E (2011) The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthr Cartil 19:1270–1285
Hochberg MC, Lethbridge-Cejku M, Tobin JD (2004) Bone mineral density and osteoarthritis: data from the Baltimore longitudinal study of aging. Osteoarthr Cartil 12:45–48
Haugen IK, Slatkowsky-Christensen B, Orstavik R, Kvien TK (2007) Bone mineral density in patients with hand osteoarthritis compared to population controls and patients with rheumatoid arthritis. Ann Rheum Dis 66:1594–1598
Zoli A, Lizzio MM, Capuano A, Massafra U, Barini A, Ferraccioli G (2006) Osteoporosis and bone metabolism in postmenopausal women with osteoarthritis of the hand. Menopause 13:462–466
Kim SK, Park SH, Choe JY (2010) Lower bone mineral density of forearm in postmenopausal patients with radiographic hand osteoarthritis. Rheumatol Int 30:605–612
Guler-Yuksel M, Bijsterbosch J, Allaart CF et al (2011) Accelerated metacarpal bone mineral density loss is associated with radiographic progressive hand osteoarthritis. Ann Rheum Dis 70:1625–1630
El-Sherif HE, Kamal R, Moawyah O (2008) Hand osteoarthritis and bone mineral density in postmenopausal women; clinical relevance to hand function, pain and disability. Osteoarthr Cartil 16:12–17
Bihlet AR, Byrjalsen I, Bay-Jensen AC et al (2019) Associations between biomarkers of bone and cartilage turnover, gender, pain categories and radiographic severity in knee osteoarthritis. Arthritis Res Ther 21:203
Kraus VB, Karsdal MA (2021) Osteoarthritis: current molecular biomarkers and the way forward. Calcif Tissue Int 109:329–338
Madry H, van Dijk CN, Mueller-Gerbl M (2010) The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc 18:419–433
Goldring SR (2012) Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Ther Adv Musculoskelet Dis 4:249–258
Burr DB, Gallant MA (2012) Bone remodeling in osteoarthritis. Nat Rev Rheumatol 8:665–673
Intema F, Hazewinkel HAW, Gouwens D et al (2010) In early OA, thinning of the subchondral plate is directly related to cartilage damage: results from a canine ACLT-meniscectomy model. Osteoarthr Cartil 18:691–698
Amir G, Pirie CJ, Rashad S, Revell PA (1992) Remodeling of subchondral bone in osteoarthritis: a histomorphometric study. J Clin Pathol 45:990–992
Kuliwaba JS, Findlay DM, Atkins GJ, Forwood MR, Fazzalari NL (2000) Enhanced expression of osteocalcin mRNA in human osteoarthritic trabecular bone of the proximal femur is associated with decreased expression of interleukin-6 and interleukin-11 mRNA. J Bone Miner Res 15:332–341
Haara MM, Heliovaara M, Kroger H (2004) Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. J Bone Joint Surg Am 86:1452–1457
Lovati AB, Pozzi A, Bongio M, Recordati C, Berzero G, Moretti M (2015) A comparative study of diagnostic and imaging techniques for osteoarthritis of the trapezium. Rheumatology (Oxford) 54:96–103
Nufer P, Goldhahn J, Kohler T, Kuhn V, Muller R, Herren DB (2008) Microstructural adaptation in trapezial bone due to subluxation of the thumb. J Orthop Res 26:208–216
Lee AT, Williams AA, Lee J, Cheng R, Lindsey DP, Ladd AL (2013) Trapezium trabecular morphology in carpometacarpal arthritis. J Hand Surg Am 38:309–315
Hong SW, Kang JH, Kim JS, Gong HS (2020) Association between forearm cortical bone properties and handgrip strength in women with distal radius fractures: a cross-sectional study. PLoS ONE 15:e0243294
Martinez H, Davarpanah M, Missika P, Celletti R, Lazzara R (2001) Optimal implant stabilization in low density bone. Clin Oral Implants Res 12:423–432
Kim SK, Jung UH, Kim JW, Choe JY (2021) Ultrasound findings were associated with radiographic changes, but not clinical and functional outcomes in hand osteoarthritis. J Rheum Dis 28:17–24
Bolbos RI, Zuo J, Banerjee S (2008) Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3T. Osteoarthr Cartil 16:1150–1159
Cao Y, Stannus OP, Aitken D et al (2014) Cross-sectional and longitudinal associations between systemic, subchondral bone mineral density and knee cartilage thickness in older adults with or without radiographic osteoarthritis. Ann Rheum Dis 73:2003–2009
Schreiber JJ, McQuillan TJ, Halilaj E et al (2018) Changes in local bone density in early thumb carpometacarpal joint osteoarthritis. J Hand Surg Am 43:33–38
Johnston JD, Kontulainen SA, Masri BA, Wilson DR (2009) A comparison of conventional maximum intensity projection with a new depth-specific topographic mapping technique in the CT analysis of proximal tibial subchondral bone density. Skeletal Radiol 39:867–876
Sniekers YH, Intema F, Lafeber FPJG et al (2008) A role for subchondral bone changes in the process of osteoarthritis; a micro-CT study of two canine models. BMC Musculoskelet Disord 9:20
Burr DB, Schaffler MB (1997) The involvement of subchondral mineralized tissues in osteoarthorsis: quantitative microscopic evidence. Microsc Res Tech 37:343–357
Bettica P, Cline G, Hart DJ, Meyer J, Spector TD (2002) Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum 46:3178–3184
Intema F, Sniekers YH, Weinans H et al (2010) Similarities and discrepancies in subchondral bone structure in two differently induced canine models of osteoarthritis. J Bone Miner Res 25:1650–1657
Cox LGE, van Donkelaar CC, van Rietbergen B, Emans PJ, Ito K (2012) Decreased bone tissue mineralization can partly explain subchondral sclerosis observed in osteoarthritis. Bone 50:1152–1161
Hwang JS, Lee S, Gong HS (2022) The impact of acute fracture on interpretation of bone turnover marker measurements for patients starting anti-resorptive therapies. Bone 154:116199
Funding
This work was supported by the SNUBH research fund granted by the Seoul National University Bundang Hospital (Grant Number 14-2021-0031), the SNUH research fund granted by the Seoul National University Hospital (Grant Number 0420220570), and the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (Grant Number 2020R1A2C1005778).
Author information
Authors and Affiliations
Contributions
Author HSG designed the study. He is guarantor. Author JSH acquired the data, analyzed and interpreted the data, and prepared the first draft of the paper. He is responsible for statistical analysis of the data. Author HSL contributed to the experimental work. All authors revised the paper critically for intellectual content and approved the final version. All authors agreed to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
Ji Sup Hwang, Han Sang Lee, Hyun Sik Gong these authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hwang, J.S., Lee, H.S. & Gong, H.S. Three-Dimensional Analysis of the Trapezium Subchondral Bone and its Association with Trapeziometacarpal Joint Osteoarthritis. Calcif Tissue Int 112, 320–327 (2023). https://doi.org/10.1007/s00223-022-01040-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01040-5