Abstract
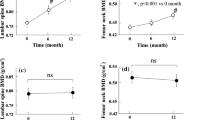
There is limited evidence on the use of romosozumab (ROMO) in the treatment of osteoporosis in patients on hemodialysis (HD); thus, we aimed to investigate this topic. This prospective, observational, single-center cohort study included 13 prior osteoporosis treatment-naïve patients on HD with osteoporosis. They first received ROMO once monthly for 12 months (210 mg; subcutaneously once every month). Thereafter, they received denosumab (DENO) for an additional 12 months (60 mg; subcutaneously once every 6 months). We examined the incidence of new fractures; treatment safety; and temporal changes in the bone mineral density (BMD), bone metabolism markers, and vascular calcification. No new cases of fractures were noted. The median one-year percentage changes (from the baseline) in the BMDs at the lumbar spine (LS), total hip (TH), and femoral neck (FN) were + 9.0%, + 2.5%, and + 4.7%, respectively. These changes were maintained for 24 months. The corresponding relative changes from the baseline to 24 months thereafter were + 14.9%, + 5.4%, and + 6.5%, respectively. The percentage changes in TH BMD and FN BMD were negatively correlated with baseline BMD. Coronary artery and thoracic aorta calcification scores increased slightly from baseline to 12 months thereafter. However, fatal events (cardiovascular disease-associated and all-cause deaths) did not occur during ROMO treatment. Effectiveness of ROMO was better in patients who had severe osteoporosis with low TH BMD, low FN BMD, and high tartrate-resistant acid phosphatase 5b level at ROMO initiation.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Group KDIGOKC-MUW (2017) KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2011(7):1–59
Messa P, Cerutti R, Brezzi B, Alfieri C, Cozzolino M (2009) Calcium and phosphate control by dialysis treatments. Blood Purif 27:360–368
Kanazawa I, Inaba M, Inoue D, Uenishi K, Saito M, Shiraki M, Suzuki A, Takeuchi Y, Hagino H, Fujiwara S, Sugimoto T (2020) Executive summary of clinical practice guide on fracture risk in lifestyle diseases. J Bone Miner Metab 38:746–758
Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, Pisoni RL (2014) High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 85:166–173
Baron R, Rawadi G (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148:2635–2643
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420
Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, Tipton B, Cai J, Deshpande R, Zhou L, Hale MD, Lightwood DJ, Henry AJ, Popplewell AG, Moore AR, Robinson MK, Lacey DL, Simonet WS, Paszty C (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25:948–959
Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M, Dwyer D, Stouch B, Thway TM, Stolina M, Ominsky MS, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ (2010) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 25:2647–2656
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med 377:1417–1427
Hsu CP, Maddox J, Block G, Bartley Y, Yu Z (2022) Influence of renal function on pharmacokinetics, pharmacodynamics, and safety of a single dose of romosozumab. J Clin Pharmacol. https://doi.org/10.1002/jcph.2050
Sato M, Inaba M, Yamada S, Emoto M, Ohno Y, Tsujimoto Y (2021) Efficacy of romosozumab in patients with osteoporosis on maintenance hemodialysis in Japan; an observational study. J Bone Miner Metab 39:1082–1090
Pietrzyk B, Smertka M, Chudek J (2017) Sclerostin: Intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med 26:1283–1291
Brandenburg VM, Verhulst A, Babler A, D’Haese PC, Evenepoel P, Kaesler N (2019) Sclerostin in chronic kidney disease-mineral bone disorder think first before you block it! Nephrol Dial Transplant 34:408–414
Tartaglione L, Pasquali M, Rotondi S, Muci ML, Covic A, Mazzaferro S (2017) Positioning novel biologicals in CKD-mineral and bone disorders. J Nephrol 30:689–699
Iwasaki Y, Kazama JJ, Fukagawa M (2017) Molecular abnormalities underlying bone fragility in chronic kidney disease. Biomed Res Int 2017:3485785
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, Ebeling PR, Adachi JD, Miyauchi A, Gielen E, Milmont CE, Libanati C, Grauer A (2019) One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Res 34:419–428
Block GA, Bone HG, Fang L, Lee E, Padhi D (2012) A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 27:1471–1479
Thongprayoon C, Acharya P, Acharya C, Chenbhanich J, Bathini T, Boonpheng B, Sharma K, Wijarnpreecha K, Ungprasert P, Gonzalez Suarez ML, Cheungpasitporn W (2018) Hypocalcemia and bone mineral density changes following denosumab treatment in end-stage renal disease patients: a meta-analysis of observational studies. Osteoporos Int 29:1737–1745
Festuccia F, Jafari MT, Moioli A, Fofi C, Barberi S, Amendola S, Sciacchitano S, Punzo G, Menè P (2017) Safety and efficacy of denosumab in osteoporotic hemodialysed patients. J Nephrol 30:271–279
Ebina K, Etani Y, Tsuboi H, Nagayama Y, Kashii M, Miyama A, Kunugiza Y, Hirao M, Okamura G, Noguchi T, Takami K, Goshima A, Miura T, Fukuda Y, Kurihara T, Okada S, Nakata K (2022) Effects of prior osteoporosis treatment on the treatment response of romosozumab followed by denosumab in patients with postmenopausal osteoporosis. Osteoporos Int. https://doi.org/10.1007/s00198-022-06386-y
Tominaga A, Wada K, Okazaki K, Nishi H, Terayama Y, Kato Y (2021) Early clinical effects, safety, and predictors of the effects of romosozumab treatment in osteoporosis patients: one-year study. Osteoporos Int 32:1999–2009
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis–executive summary. Arch Osteoporos 7:3–20
Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T, Group C-MGW, Japanese Society for Dialysis T (2013) Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 17:247–288
Nishizawa Y, Miura M, Ichimura S, Inaba M, Imanishi Y, Shiraki M, Takada J, Chaki O, Hagino H, Fukunaga M, Fujiwara S, Miki T, Yoshimura N, Ohta H (2019) Executive summary of the Japan osteoporosis society guide for the use of bone turnover markers in the diagnosis and treatment of osteoporosis (2018 edition). Clin Chim Acta 498:101–107
Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, D’Haese PC, Drüeke TB, Du H, Manley T, Rojas E, Moe SM (2016) Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD Treated by Dialysis. Am J Kidney Dis 67:559–566
Shidara K, Inaba M, Okuno S, Yamada S, Kumeda Y, Imanishi Y, Yamakawa T, Ishimura E, Nishizawa Y (2008) Serum levels of TRAP5b, a new bone resorption marker unaffected by renal dysfunction, as a useful marker of cortical bone loss in hemodialysis patients. Calcif Tissue Int 82:278–287
Ebina K, Hirao M, Hashimoto J, Matsuoka H, Iwahashi T, Chijimatsu R, Etani Y, Okamura G, Miyama A, Yoshikawa H (2018) Impact of switching oral bisphosphonates to denosumab or daily teriparatide on the progression of radiographic joint destruction in patients with biologic-naïve rheumatoid arthritis. Osteoporos Int 29:1627–1636
Baim S, Wilson CR, Lewiecki EM, Luckey MM, Downs RW Jr, Lentle BC (2005) Precision assessment and radiation safety for dual-energy X-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom 8:371–378
Budoff MJ, Achenbach S, Fayad Z, Berman DS, Poon M, Taylor AJ, Uretsky BF, Williams KA (2006) Task Force 12: training in advanced cardiovascular imaging (computed tomography): endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Atherosclerosis Imaging and Prevention, and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 47:915–920
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–832
Miller PD, Adachi JD, Albergaria BH, Cheung AM, Chines AA, Gielen E, Langdahl BL, Miyauchi A, Oates M, Reid IR, Santiago NR, Vanderkelen M, Wang Z, Yu Z (2022) Efficacy and safety of romosozumab among postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J Bone Miner Res
Ebina K, Hirao M, Tsuboi H, Nagayama Y, Kashii M, Kaneshiro S, Miyama A, Nakaya H, Kunugiza Y, Okamura G, Etani Y, Takami K, Goshima A, Nakata K (2020) Effects of prior osteoporosis treatment on early treatment response of romosozumab in patients with postmenopausal osteoporosis. Bone 140:115574
Shimizu T, Arita K, Murota E, Hiratsuka S, Fujita R, Ishizu H, Asano T, Takahashi D, Takahata M, Iwasaki N (2021) Effects after starting or switching from bisphosphonate to romosozumab or denosumab in Japanese postmenopausal patients. J Bone Miner Metab 39:868–875
Tominaga A, Wada K, Kato Y, Nishi H, Terayama Y, Okazaki K (2021) Early clinical effects, safety, and appropriate selection of bone markers in romosozumab treatment for osteoporosis patients: a 6-month study. Osteoporos Int 32:653–661
Ha L, Shi JB, Yu HY, Yang K, Wang HN, Wang FF, Han JL (2020) Association between serum cartilage oligomeric matrix protein and coronary artery calcification in maintenance hemodialysis patients. J Geriatr Cardiol 17:67–73
Vervloet M, Cozzolino M (2017) Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int 91:808–817
Zhang K, Gao J, Chen J, Liu X, Cai Q, Liu P, Huang H (2016) MICS, an easily ignored contributor to arterial calcification in CKD patients. Am J Physiol Renal Physiol 311:F663-f670
Viaene L, Behets GJ, Heye S, Claes K, Monbaliu D, Pirenne J, D’Haese PC, Evenepoel P (2016) Inflammation and the bone-vascular axis in end-stage renal disease. Osteoporos Int 27:489–497
Drechsler C, Evenepoel P, Vervloet MG, Wanner C, Ketteler M, Marx N, Floege J, Dekker FW, Brandenburg VM (2015) High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant 30:288–293
Gong L, Zheng D, Yuan J, Cao L, Ni Z, Fang W (2018) Elevated levels of serum sclerostin are linked to adverse cardiovascular outcomes in peritoneal dialysis patients. Int Urol Nephrol 50:955–961
Malluche HH, Monier-Faugere MC, Blomquist G, Davenport DL (2018) Two-year cortical and trabecular bone loss in CKD-5D: biochemical and clinical predictors. Osteoporos Int 29:125–134
Evenepoel P, Cunningham J, Ferrari S, Haarhaus M, Javaid MK, Lafage-Proust MH, Prieto-Alhambra D, Torres PU, Cannata-Andia J (2021) Diagnosis and management of osteoporosis in chronic kidney disease stages 4 to 5D: a call for a shift from nihilism to pragmatism. Osteoporos Int 32:2397–2405
Acknowledgements
The authors thank Ms. Miyoko Watanabe and the other staff members at the Sannoudai hospital for their assistance with data collaboration, data monitoring, and the conceptualization and execution of this study.
Funding
This study received no external funding.
Author information
Authors and Affiliations
Contributions
Tomohiro Saito contributed to the study design, performed the study and data analysis, drafted the main manuscript, and provided the final approval for submission. Yasuro Fujiwara contributed to the study design and performed the study. Naoaki Kanamori contributed to the study design and performed the study. Masahide Mizobuchi reviewed the manuscript critically for important intellectual content and provided the final approval for submission. Mikio Makuuchi reviewed the manuscript critically for important intellectual content and provided the final approval for submission. Hirokazu Honda reviewed the manuscript critically for important intellectual content and provided the final approval for submission.
Corresponding author
Ethics declarations
Competing interests
Tomohiro Saito, Masahide Mizobuchi, Tadashi Kato, Taihei Suzuki, Yasuro Fujiwara, Naoaki Kanamori, Mikio Makuuchi, and Hirokazu Honda declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The study protocol was approved by the ethical committee of the Showa University Hospital (No. 2896) and registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR ID: UMIN000039889). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants gave written informed consent. No animal studies were performed in the course of these experiments.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saito, T., Mizobuchi, M., Kato, T. et al. One-Year Romosozumab Treatment Followed by One-Year Denosumab Treatment for Osteoporosis in Patients on Hemodialysis: An Observational Study. Calcif Tissue Int 112, 34–44 (2023). https://doi.org/10.1007/s00223-022-01031-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01031-6