Abstract
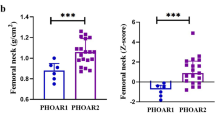
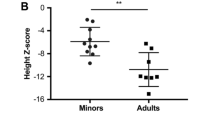
Achondroplasia (ACH) is a skeletal disorder caused by fibroblast growth factor receptor 3 (FGFR3) variants. Volumetric bone mineral density (vBMD), bone microarchitecture, and strength have not been evaluated in these patients previously. This study aims to evaluate vBMD, bone microarchitecture, and strength in ACH patients. Seventeen patients underwent clinical and biochemical evaluations, and genetic testing. High-resolution peripheral quantitative computed tomography was performed in 10 ACH patients and 21 age- and sex-matched healthy subjects. All individuals had the hotspot mutation of c.1138G > A in FGFR3. Linear growth retardation, disproportionate short stature, and genu varum are the most common manifestations. The mean height was 108.82 ± 24.08 cm (Z score: − 5.72 ± 0.96). Total vBMD in the ACH and the control groups was 427.08 ± 49.29 mg HA/cm3 versus 300.35 ± 69.92 mg HA/cm3 (p < 0.001) at the radius and 336.90 ± 79.33 mg HA/cm3 versus 292.20 ± 62.35 mg HA/cm3 (p = 0.098) at the tibia; both at the radius and tibia, vBMD of trabecular bones was significantly lower in the ACH group than in the control group, but vBMD of cortical bones was slightly higher in the ACH group. Trabecular separation and cortical thickness in the ACH group were significantly higher than those in the control group, but trabecular number was significantly decreased in the ACH group. Stiffness and failure load were only better at the radius in the ACH group. ACH patients have higher total and cortical vBMD, lower trabecular vBMD, worse trabecular bone microarchitecture, thicker cortical bone thickness, and better estimated bone strength.


Similar content being viewed by others
Data Availability
The raw datasets generated and/or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.
References
Ornitz DM, Legeai-Mallet L (2017) Achondroplasia: development, pathogenesis, and therapy. Dev Dyn 246:291–309
Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ (1994) Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78:335–342
Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer M, Munnich A (1994) Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature 371:252–254
Pauli RM (2019) Achondroplasia: a comprehensive clinical review. Orphanet J Rare Dis 14:1
Coi A, Santoro M, Garne E, Pierini A, Addor MC, Alessandri JL, Bergman JEH, Bianchi F, Boban L, Braz P, Cavero-Carbonell C, Gatt M, Haeusler M, Klungsøyr K, Kurinczuk JJ, Lanzoni M, Lelong N, Luyt K, Mokoroa O, Mullaney C, Nelen V, Neville AJ, O’Mahony MT, Perthus I, Rankin J, Rissmann A, Rouget F, Schaub B, Tucker D, Wellesley D, Wisniewska K, Zymak-Zakutnia N, Barišić I (2019) Epidemiology of achondroplasia: a population-based study in Europe. Am J Med Genet A 179:1791–1798
Legare JM (1993) Achondroplasia. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A (eds) GeneReviews(®). University of Washington, Seattle
McDonald EJ, De Jesus O (2021) Achondroplasia. In: StatPearls. StatPearls Publishing, Treasure Island
Högler W, Ward LM (2020) New developments in the management of achondroplasia. Wien Med Wochenschr 170:104–111
Arita ES, Pippa MG, Marcucci M, Cardoso R, Cortes AR, Watanabe PC, Oliveira JX (2013) Assessment of osteoporotic alterations in achondroplastic patients: a case series. Clin Rheumatol 32:399–402
Matsushita M, Kitoh H, Mishima K, Kadono I, Sugiura H, Hasegawa S, Nishida Y, Ishiguro N (2016) Low bone mineral density in achondroplasia and hypochondroplasia. Pediatr Int 58:705–708
Sims D, Onambélé-Pearson G, Burden A, Payton C, Morse C (2019) Whole-body and segmental analysis of body composition in adult males with achondroplasia using dual X-ray absorptiometry. PLoS ONE 14:e0213806
Carey JJ, Delaney MF (2017) Utility of DXA for monitoring, technical aspects of DXA BMD measurement and precision testing. Bone 104:44–53
Adams JE (2013) Advances in bone imaging for osteoporosis. Nat Rev Endocrinol 9:28–42
Nishiyama KK, Shane E (2013) Clinical imaging of bone microarchitecture with HR-pQCT. Curr Osteoporos Rep 11:147–155
Dash AS, Agarwal S, McMahon DJ, Cosman F, Nieves J, Bucovsky M, Guo XE, Shane E, Stein EM (2020) Abnormal microarchitecture and stiffness in postmenopausal women with isolated osteoporosis at the 1/3 radius. Bone 132:115211
Mendes DAB, Coelho MCA, Gehrke B, de Pinho LKJ, Cardoso Lima LF, Paranhos-Neto F, de Mendonça LMC, Farias MLF, Madeira M (2021) Microarchitectural parameters and bone mineral density in patients with tumour-induced osteomalacia by HR-pQCT and DXA. Clin Endocrinol 95:587–594
Folkestad L, Groth KA, Shanbhogue V, Hove H, Kyhl K, Østergaard JR, Jørgensen NR, Andersen NH, Gravholt CH (2020) Bone geometry, density, and microarchitecture in the distal radius and tibia in adults with Marfan syndrome assessed by HR-pQCT. J Bone Miner Res 35:2335–2344
Li H, Ji C, Zong X, Zhang Y (2009) Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Chin J Pediatr 47:487–492
MacNeil JA, Boyd SK (2008) Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 30:792–799
Yu F, Xu Y, Hou Y, Lin Y, Jiajue R, Jiang Y, Wang O, Li M, Xing X, Zhang L, Qin L, Hsieh E, Xia W (2020) Age-, site-, and sex-specific normative centile curves for HR-pQCT-derived microarchitectural and bone strength parameters in a Chinese Mainland population. J Bone Miner Res 35:2159–2170
Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK (2007) Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 41:505–515
Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK (2010) Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone 47:519–528
Manske SL, Zhu Y, Sandino C, Boyd SK (2015) Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone 79:213–221
Hildebrand T, Laib A, Müller R, Dequeker J, Rüegsegger P (1999) Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14:1167–1174
Pistoia W, van Rietbergen B, Lochmüller EM, Lill CA, Eckstein F, Rüegsegger P (2002) Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone 30:842–848
Ajmal M, Mir A, Shoaib M, Malik SA, Nasir M (2017) Identification and in silico characterization of p.G380R substitution in FGFR3, associated with achondroplasia in a non-consanguineous Pakistani family. Diagn Pathol 12:47
Liang H, Qi W, Jin C, Pang Q, Liu W, Jiang Y, Wang O, Li M, Xing X, Pan H, Xia W. Data from: evaluation of volumetric bone mineral density, bone microarchitecture and bone strength in patients with achondroplasia caused by FGFR3 c.1138G>A mutation. figshare. Dataset. https://doi.org/10.6084/m9.figshare.19071713.v1
Klag KA, Horton WA (2016) Advances in treatment of achondroplasia and osteoarthritis. Hum Mol Genet 25:R2-8
Daugherty A (2017) Achondroplasia: etiology, clinical presentation, and management. Neonatal Netw 36:337–342
Han J, Yang YD, He Y, Liu WJ, Zhen L, Pan M, Yang X, Zhang VW, Liao C, Li DZ (2020) Rapid prenatal diagnosis of skeletal dysplasia using medical trio exome sequencing: benefit for prenatal counseling and pregnancy management. Prenat Diagn 40:577–584
Dai WQ, Gu XF, Yu YG (2020) Exploring the clinical genetic characteristics and height for age growth curve of 210 patients with achondroplasia in China. Zhonghua Er Ke Za Zhi 58:461–467
Rao GN, Greger NG, Nelson DA (1999) Whole body bone mass and body composition in a girl with achondroplasia, at ages 9 through 12. J Clin Densitom 2:185–190
de Vries OM, Johansen H, Fredwall SO (2021) Physical fitness and activity level in Norwegian adults with achondroplasia. Am J Med Genet A 185:1023–1032
Okura T, Matsushita M, Mishima K, Esaki R, Seki T, Ishiguro N, Kitoh H (2018) Activated FGFR3 prevents subchondral bone sclerosis during the development of osteoarthritis in transgenic mice with achondroplasia. J Orthop Res 36:300–308
Chen H, Sun X, Yin L, Chen S, Zhu Y, Huang J, Jiang W, Chen B, Zhang R, Chen L, Nie M, Xie Y, Deng Z (2017) PTH 1–34 ameliorates the osteopenia and delayed healing of stabilized tibia fracture in mice with achondroplasia resulting from gain-of-function mutation of FGFR3. Int J Biol Sci 13:1254–1265
Katsimbri P (2017) The biology of normal bone remodelling. Eur J Cancer Care 26:e12740
Rowe P, Koller A, Sharma S (2021) Physiology, bone remodeling. In: StatPearls. StatPearls Publishing, Treasure Island
Acknowledgements
We are so grateful to all of the participants in this study.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81970757), the Chinese National Key Technology R & D Program, Ministry of Science and Technology (Grant No. 2021YFC2501700), and the Chinese Academy of Medical Sciences-CAMS Innovation Fund for Medical Sciences (Grant No. CIFMS-2021-12M-1-002).
Author information
Authors and Affiliations
Contributions
WBX designed the study and revised the manuscript. HTL and QQP analyzed the genetic results. HTL analyzed all data and draft the manuscript. WTQ and CXJ collected HR-pQCT data. WL, YJ, OW, ML, XPX, HP, and WBX collected clinical information of patients. HTL and WBX are responsible for the integrity of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Hanting Liang, Wenting Qi, Chenxi Jin, Qianqian Pang, Wei Liu, Yan Jiang, Ou Wang, Mei Li, Xiaoping Xing, Hui Pan, and Weibo Xia declares no competing interest.
Ethical Approval
The study was performed with the approval of the Ethics Committee of Peking Union Medical College Hospital (JS-1689).
Informed Consent
All of the subjects agreed to participate in this study and signed informed consent forms.
Consent to Publication
All of the authors agreed to publish the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, H., Qi, W., Jin, C. et al. Evaluation of Volumetric Bone Mineral Density, Bone Microarchitecture, and Bone Strength in Patients with Achondroplasia Caused by FGFR3 c.1138G > A Mutation. Calcif Tissue Int 112, 13–23 (2023). https://doi.org/10.1007/s00223-022-01027-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01027-2